PRODUCT IDENTIFICATION
203-606-5
H.S. CODE
TOXICITY
CLASSIFICATION
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
222 C
1.115
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
109 C
Stable under ordinary conditions. Hygroscopic. air sensitive; light sensitive.
EXTERNAL LINKS & GENERAL DESCRIPTION
PubChem Compound Summary - 3,5-Dimethylphenol
Local: Cresols are methyl substituted phenols at relative to the hydroxyl group, ortho-, meta-, and para-cresol. There are three structural isomers. The names of the three compounds indicate which of the hydrogens on the benzene ring portion of the molecule have been replaced. They are obtained from coal tar or petroleum as by-products in the fractional distillation and in coal gasification. They are also formed as by-products during the combustion of wood. The various isomers can be manufactured by the methylation of phenol, toluene sulfonation and alkaline hydrolysis, or the hydrolysis of 2-isopropyltoluene or alkaline chlorotoluene. Because the boiling points of these three compounds are nearly the same, a separation of a mixture of the three into its pure components is impractical. They are highly flammable and soluble in water, ethanol, ether, acetone and alkali hydroxides. The mixture of cresols obtained from coal tar is called cresylic acid, an important technical product used as a disinfectant and in the manufacture of resins and tricresyl phosphate. Cresylic acid also refs to the mixture of phenols containing varying amounts of xylenols, cresols, and other high-boiling fractions, but not more than 5 percent phenol. Commercial cresols are prepared in a wide range of grades and purities to meet the user's requirements. It is a liquid from clear to brown and is toxic to animals including human. It is corrosive and is a more powerful disinfectant and antiseptic than phenol. The primary use is for sterilizing as disinfectants and deodorizers, and pesticides. Its solution is used as household cleaners as a disinfectant. Creosote products are mixtures of many aromatic hydrocarbons including phenols and cresols. Creosote obtained from coal tar is poisonous and provides protection against fungi, shipworms, termites, and psoriasis. It is used chiefly as a wood preservative, e.g., in wooden poles, railroad ties, and timber. They are also used as animal and bird repellents. Animals may suffer skin irritation or ulceration from creosote treated wood. Coal tar creosote and its derivatives are the most widely used wood preservatives. Wood tar creosote is a mixture of chiefly guaiacol, creosols and other phenolic compounds obtained from wood tar (mainly beech) by distillation between 203 and 220 C. It is insoluble in water, soluble in methanol, acetone. It is used as an external antiseptic, expectorant, gastric sedative, deodorant, and as an antiseptic parasiticide veterinary use in the form of creosote carbonate. It is used in the synthesis of pharmaceuticals and vanillin. Each cresols are used as solvents or disinfectants and as useful as raw materials for various chemical products including;
- Antiseptics, disinfectants
- Fragrances, deodorizing, odor-enhancer
- Resins (phenol-formaldehyde, phenolic, and epoxy) and their additives
- Phosphate esters (plasticizers)
- Herbicides and pharmaceuticals
- Rubber and plastic antioxidants
- Dyes and pigments
- Household cleaners and automotive degreasers
- Solvent and paints
- Lubricating oils, gasoline additives
- Adhesives
- Fiber and wood preservatives
- UV- absorbers and photographic chemicals
- Ore flotation agents
Cresols undergo electrophilic substitution reactions such as chlorination, bromination, sulfonation and nitration at the vacant position. They also undergo condensation reactions with aldehydes, ketones or dienes. O-cresol is a starting material for the synthesis of herbicides such as 4,6-dinitro-o-cresol (DNOC) and 2-methyl-4-chlorophenoxyacetic acid (MCPA). Meta-cresol is used in the manufacture of explosives. Meta and para-cresol are used in phenol-formaldehyde resins and are converted to tricresyl phosphate used as a plasticizer and gasoline additive and antioxidants such as di-tert-butylcresols (BHT). Ortho- and para-cresols are used in the production of lubricating oils and motor fuels.
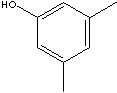
Xylenols, dimethylphenol, are isomeric substituted phenols with two methyl substituent at either the ortho-, meta- or para position relative to the hydroxyl group. They have similar characteristics and applications with cresols.
Isomers:
|
Xylenol |
CAS RN |
M.P C |
B.P C |
| 2,3-Xylenol | 526-75-0 | 70-73 | 217 |
| 2,4-Xylenol | 105-67-9 | 25-26 | 211-212 |
| 2,5-Xylenol | 95-87-4 | 63 - 65 | 212 |
|
2,6-Xylenol |
576-26-1 |
43-45 |
203 |
| 3,4-Xylenol | 95-65-8 | 62 - 68 | 227 |
| 3,5-Xylenol | 108-68-9 | 64 | 219 |
APPEARANCE
ASSAY (G.C)
99.0% min
MOISTURE (K.F.)
0.1% max
ISOMER IMPURITY
0.5% max
HAZARD OVERVIEW
Toxic by skin absorption, Corrosive, Target Organ Effect, Toxic by ingestion.
GHS
PICTOGRAMS


HAZARD STATEMENTS
H314 Causes severe skin burns and eye damage
H311 Toxic
in contact with skin
H301 Toxic if swallowed
PRECAUTIONARY STATEMENTS
P280 Wear protective gloves/protective clothing/eye protection/face
protection
P312 Call a POISON CENTER or doctor/physician
if you feel unwell
P302 + P350 IF ON SKIN: Gently wash
with plenty of soap and water
P301 + P330 + P331 IF SWALLOWED:
rinse mouth. Do NOT induce vomiting
P305 + P351 + P338 IF
IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing
P301+
P312 IF SWALLOWED: Call a POISON CENTER or doctor/physician
if you feel unwell
RISK PHRASES
24/25 Toxic in contact with skin and if swallowed
34
Causes burns
SAFETY PHRASES
26 In case of contact with eyes, rinse immediately with
plenty of water and seek medical advice.28A: After contact
with skin, wash immediately with plenty of water.
36/37/39 Wear
suitable protective clothing, gloves and eye/face protection.
45
In case of accident or if you feel unwell, seek medical advice
immediately (show the label where possible).