PRODUCT IDENTIFICATION
200-718-6
H.S. CODE
TOXICITY
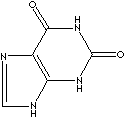
Xanthine; 1H-Purine-2,6-diol; 2,6(1,3)-Purinedion;
2,6-Dioxo-1,2,3,6-tetrahydropurine; 2,6-Dioxopurine; 3,7-Dihydro-1H-purine-2,6-dione; 9H-Purine-2,6-(1H,3H)-dione; 9H-Purine-2,6-diol; Isoxanthine; Pseudoxanthine; Purine-2,6-(1H,3H)-dione; Purine-2,6-diol; XAN; X; Xanthic oxide; Xanthin; Other RN: 6050-36-8, 6053-41-4, 16819-86-6, 28522-58-9, 33669-67-9, 42911-15-9, 51953-26-5
CLASSIFICATION
Nucleotide
EXTRA NOTES
EPA Pesticide Chemical Code 116900.
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Xanthine
PubChem Compound Summary - Xanthine
Drug Bank - Xanthine
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Xanthine
http://www.ebi.ac.uk/chebi/ - Xanthine
http://www.ncbi.nlm.nih.gov/ - Xanthine
Human Metabolome Database - Xanthine
http://www.sciencedirect.com/
The methylxanthine caffeine has many pharmacological effects, most of which can
be linked to blockade of adenosine receptors, inhibition of phosphodiesterases,
and augmentation of calcium-dependent release of calcium from intracellular
stores. A variety of xanthines have been developed as potent and/or
selective antagonists for adenosine receptors. Several xanthines have been
developed that are more potent and more selective inhibitors of cyclic
nucleotide phosphodiesterase than caffeine or theophylline. Caffeine remains
the xanthine of choice for activation of intracellular calcium-sensitive calcium
release channels although millimolar concentrations are required, which can have
effects on other aspects of calcium regulation.
Local:
Xanthine, also known as dioxopurine is a a precursor of uric acid and is
found in blood and urine, muscle tissue and in certain plants. It is a toxic
yellowish white powder insoluble in water and acids but soluble in caustic soda;
sublimes when heated. It is involved in purine degradation and
is converted to uric acid by xanthine oxidase. Purine is a heterocyclic compound
featured by a fused pyrimidine and imidazole rings composed of carbon and
nitrogen atoms. The simplest one is purine itself and the two major purines are
adenine (6-Aminopurine) and guanine (2-Amino-6-hydroxypurine). Other important
purines are caffeine, uric acid, theobromine, and theophylline. Purine and its
derivatives are biologically important components of nucleic acids (DNA, RNA)
and coenzymes. Xanthine and its analogues are used in medicine as a chemical
intermediate.
|
APPEARANCE
98.0 - 102.0%
HAZARD OVERVIEW
Causes eye, skin, and respiratory tract irritation. Target Organs: Kidneys, respiratory system, eyes, skin.
GHS
PICTOGRAMS

HAZARD STATEMENTS
H315:
Causes skin irritation
H319: Causes serious eye irritation
H335:
May cause respiratory irritation
PRECAUTIONARY STATEMENTS
P261:
Avoid breathing dust/fume/gas/mist/vapors/spray
P280: Wear
protective gloves/protective clothing/eye protection/face protection
P302+
P352: IF ON SKIN: Wash with plenty of soap and water
P305
+ P351 + P338: IF IN EYES: Rinse cautiously with water for
several minutes. Remove contact lenses, if present and easy to do.
Continue rinsing
![]() Xi
Irritant
Xi
Irritant
RISK PHRASES
36/37/38: Irritating to eyes, respiratory system and skin.
SAFETY PHRASES
26:
In case of contact with eyes, rinse immediately with plenty
of water and seek medical advice.
37/39: Wear suitable
gloves and eye/face protection