| VALPROATE SODIUM | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 1069-66-5 |
|
| EINECS NO. | 213-961-8 | |
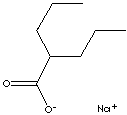
| FORMULA | C8H15O2Na | |
| MOL WT. | 166.20 | |
|
H.S. CODE |
2915.90.0090 |
|
|
TOXICITY |
Oral rat LD50: 670 mg/kg | |
| SYNONYMS | Valproic acid sodium salt; Sodium valproate; | |
| 2-propylvaleric acid, sodium salt; 2-Propylpentanoic acid sodium salt; Depakene; Dipropylacetate sodium; Epilim; Sodium 2-propylpentanoate; Sodium 2-propylvalerate; Sodium alpha,alpha-dipropylacetate; Sodium bispropylacetate; Sodium dipropylacetate; Sodium n-dipropylacetate; Valerin; Valproate sodium; sodium hydrogen bis(2-propylpentanoate); | ||
SMILES |
C(CCC)(CCC)C(=O)[O-].[Na+] |
|
|
CLASSIFICATION |
Anticonvulsant; Antiepileptic; Antimanic agent; Central Nervous System Depressant; Enzyme inhibitor; GABA agent; Neurotransmitter Agent; Psychotropic Drug; Tranquilizing Agent; | |
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
white to off-white crystalline powder, odorless | |
| MELTING POINT | ||
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | Freely soluble in water | |
|
SOLVENT SOLUBILITY |
Soluble in alcohol | |
| pH | ||
| VAPOR DENSITY |
| |
|
AUTOIGNITION |
| |
|
NFPA RATINGS |
||
|
REFRACTIVE INDEX |
||
| FLASH POINT |
| |
| STABILITY | Stable under ordinary conditions. | |
|
GENERAL DESCRIPTION & EXTERNAL LINKS |
||
|
Valproic acid and its derivatives are used solely or in combination with other anticonvulsants to prevent seizures and migraines. Valproates are effective against the manic phase of bipolar disorder. A fatty acid with anticonvulsant properties used in the treatment of epilepsy. The mechanisms of its therapeutic actions are not well understood. It may act by increasing GAMMA-AMINOBUTYRIC ACID levels in the brain or by altering the properties of voltage dependent sodium channels. Wikipedia Linking:http://en.wikipedia.org/wiki/Sodium_valproate http://www.merck.com/ http://www.ncbi.nlm.nih.gov/ |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white powder |
|
|
CONTENT |
98.0% min |
|
| TRANSPORTATION | ||
| PACKING |
| |
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION (LIST OF ANTICONVULSANTS) | ||