PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
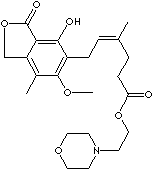
Mycophenolate mofetil; Mycophenolic acid 2-morpholinoethyl ester;
C1OCCN(C1)CCOC(CCC(\C)=C/Cc1c(c(c2c(c1O)C(OC2)=O)C)OC)=O
CLASSIFICATION
Immunomodulator
EXTRA NOTES
Mycophenolic acid that is chemically altered by the addition of morpholinoethylester; selective and reversible inhibitor of inosine monophosphate dehydrogenase.
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
Health hazard: 2, Flammability: 0, Physical hazards: 0
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Mycophenolate mofetil
PubChem Compound Summary - Mycophenolate mofetil
Drug Bank - Mycophenolic acid
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Mycophenolate mofetil
http://www.ebi.ac.uk/ - Mycophenolate mofetil
http://www.ncbi.nlm.nih.gov/ - Mycophenolate mofetil
http://www.sigmaaldrich.com/Mycophenolate mofetil is a prodrug of mycophenolic acid that is cleaved by nonspecific esterases in vivo to produce the parent compound. Mycophenolic acid blocks inosine monophosphate dehydrogenase and is a potent immunosuppresive agent.
http://www.gene.com/
Mycophenolate
mofetil has been demonstrated in experimental animal models to prolong
the survival of allogeneic transplants (kidney, heart, liver, intestine,
limb, small bowel, pancreatic islets, and bone marrow). Mycophenolate
mofetil has also been shown to reverse ongoing acute rejection in
the canine renal and rat cardiac allograft models. Mycophenolate
mofetil also inhibited proliferative arteriopathy in experimental
models of aortic and cardiac allografts in rats, as well as in primate
cardiac xenografts. Mycophenolate mofetil was used alone or in combination
with other immunosuppressive agents in these studies. Mycophenolate
mofetil has been demonstrated to inhibit immunologically mediated
inflammatory responses in animal models and to inhibit tumor development
and prolong survival in murine tumor transplant models.
Local:
Mycophenolate, 2-morpholinoethyl ester of mycophenolic acid, is a guanine
monophosphate synthesis inhibitor by interfering with the synthesis of inosine
mononophosphate dehydrogenase (IMPDH), resulting in inhibiting the proliferation
of cells, especially leukocytes and lymphocytes. It is more
lymphocyte-specific than azathioprine. Mycophenolate is an immunosuppressive
agent used in conjunction with cyclosporine and corticosteroids to prevent
rejection of allogeneic renal transplants. Mycophenolate mofetil is a white to off-white crystalline powder; soluble in
acetone and methanol, and sparingly soluble in water and ethanol; administered
orally. The chemical designation is
2-morpholinoethyl
(E)-6-(l,3-dihydro-4-hydroxy-6-methoxy-7- methyl-3-oxo-5-isobenzofuranyl)-4-
methyl-4-hexenoate.
APPEARANCE
IDENTIFICATION
Complies
ASSAY (HPLC)
LOSS ON DRYING
2.0% max
20ppm max
- Corticosteroids
- Dexamethasone
- Prednisolone
- Prednisone
- Polyclonal antibodies
- Antilymphocyte globulin
- Antithymocyte globulin
- Macrolides
- Sirolimus
- Tacrolimus
- Antimetabolites
- Azathioprine
- Cyclophosphamide
- Methotrexate
- Monoclonal antibodies
- Basiliximab
- Daclizumab
- Infliximab
- Muromonab
- Fungal metabolite
- Cyclosporine
- Others
- Glatiramer acetate
- Mycophenolate mofetil
- Interferons
- Opioids
- Tumor necrosis factor (TNF) inhibitors
HAZARD OVERVIEW
Toxic by ingestion
GHS
PICTOGRAMS


HAZARD STATEMENTS
H302-H400
P STATEMENTS
P273