| MERCAPTOPURINE MONOHYDRATE | |||
|
PRODUCT IDENTIFICATION |
|||
| CAS NO. | 50-44-2 (Base) 6112-76-1 (Monohydrate) |
|
|
| EINECS NO. | 200-037-4 | ||
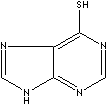
| FORMULA | C5H4N4S·H2O | ||
| MOL WT. | 170.19 | ||
|
H.S. CODE |
2933.59.9590 |
||
|
TOXICITY |
Mouse LD50 (Oral): 1250mg/kg | ||
| SYNONYMS | 1,7-Dihydro-6H-purine-6-thione monohydrate; | ||
| 6-Purinethiol; 6-Thioxopurine monohydrate; 7-Mercapto-1,3,4,6-tetrazaindene monohydrate; Thiohypoxanthine, monohydrate; Purimethol monohydrate; Purine-6-thiol monohydrate; Purinethiol monohydrate; 6-Thiohypoxanthine monohydrate; Other RN: 5759-99-9; 5818-33-7; 5818-60-0; 39454-94-9; | |||
|
SMILES |
c12c(nc[nH]c1=S)nc[nH]2.O | ||
|
CLASSIFICATION |
Nucleoside, Purine, Antimetabolite, Antileukemic agent, Antineoplastic, Enzyme Inhibitor, Immunologic Factor, Immunosuppressive agent, Nucleic acid synthesis inhibitor | ||
|
PHYSICAL AND CHEMICAL PROPERTIES |
|||
| PHYSICAL STATE |
yellow powder |
||
| MELTING POINT | >313 C | ||
| BOILING POINT | |||
| SPECIFIC GRAVITY | |||
| SOLUBILITY IN WATER | Insoluble (soluble in diluted alkali solutions slightly soluble in diluted sulfuric acid soluble in hot alcohol insoluble in acetone and ether) | ||
| pH | |||
| VAPOR DENSITY |
|
||
| log Pow | 0.01 (Octanol-water) | ||
| VAPOR PRESSURE | 1.43E-06 (mmHg at 25 C) | ||
| HENRY'S LAW | 7.49E-10 (atm-m3/mole at 25 C) | ||
| OH RATE | 2.00E-10 (cm3/molecule-sec at 25 C Atmospheric) | ||
| AUTOIGNITION |
|
||
| NFPA RATINGS | |||
|
REFRACTIVE INDEX |
|||
| FLASH POINT | |||
| STABILITY |
Stable under ordinary conditions. |
||
|
GENERAL DESCRIPTION & APPLICATIONS |
|||
| Purine is a heterocyclic compound featured by a fused pyrimidine and imidazole
rings composed of carbon and nitrogen atoms. The simplest one is purine itself
and the two major purines are adenine(6-Aminopurine) and
guanine(2-Amino-6-hydroxypurine) which are two bases components of nucleic acid
and the nucleotides. Purine itself is not found in nature, but as substituted
purines such as methyled, hydroxyl and amino substituted. In addition to adenine
and guanine, a group of chemical compounds called purine base include
hypoxanthine (6-oxypurine), xanthine (2,6-dioxypurine), uric acid
(2,6,8-trioxypurine), and theobromine (3,7-dimethyl xanthine). Theophylline and
caffeine are a member of methylated purine family. Purines are biologically
important in In medicine and biological research.
Mercaptopurine (6-MP), a purine analogue in which sulfur replaces the oxygen atom of purine, interfere with nucleic acid biosynthesis and used as an antineoplastic. 6-MP is an antagonist used in cancer chemotherapy for the treatment of acute lymphocytic leukemia, acute myelogenous leukemia and chronic myelogenous leukemia. 6-MP is cross-resistant with 6-thioguanine. It is incorporated into 6-thioinosine monophosphate which inhibits de novo purine synthesis in two places, by serving as a pseudofeedback inhibitor of the first step in the pathway and also by inhibiting the conversion of IMP to adenine and guanine nucleotides. Cytotoxicity is cell cycle phase-specific (S-phase). It is also used as an immunosuppressive in the treatment of Crohn's disease, ulcerative colitis, severe psoriatic arthritis, and polycythemia vera. It interferes with nucleic acid synthesis by inhibiting purine metabolism and is used, usually in combination with other drugs, in the treatment of or in remission maintenance programs for leukemia. Antimetabolite is a chemical compound that closely resembles a metabolite in molecular structure, but can interfering with the normal reactions of the essential metabolite. It prevent the cell from the utilization of the genuine substance necessary to normal biochemical reactions. Chemically modified nucleotides substituted or attached by special chemical groups or elements are studied and used to inactivate the normal biological operation in the living organism and the function of important enzymes. Aminopterin (4-aminofolic acid) and methotrexate (mthylaminopterin) are antagonists of folic acid, and used in the treatment of some neoplastic diseases. Azathioprine and mercaptopurine are purine antagonists. 5-Fluorouracil and fluorodeoxyuridine are examples of pyrimidine antagonists; used as an antineoplastic and as a component of chemotherapy regimens. DNA synthesis inhibitors for antitumor effects include:
Wikipedia Linking: http://en.wikipedia.org/wiki/Mercaptopurine Information for Mercaptopurine from WorldWideScience.org: http://worldwidescience.org/ |
|||
| SALES SPECIFICATION | |||
|
APPEARANCE |
yellow crystalline powder | ||
|
IDENTIFICATION |
Complies Test A,B |
||
| PURITY |
97.0 - 102.0% |
||
| TOTAL IMPURITY |
1.0% max |
||
|
HEAVY METALS |
10ppm max |
||
|
LOSS ON DRYING |
12.0% max |
||
| HYPOXANTHINE | 0.5% max | ||
| TRANSPORTATION | |||
| PACKING |
|
||
| HAZARD CLASS | |||
| UN NO. | |||
| SAFETY INFORMATION | |||
Hazard Symbols: XN, Risk Phrases: 22-36/37/38, Safety Phrases: 22-36/37/39-45 |
|||