Pyridinecarboxylic acids are active in the metabolism of body. For example,
nicotinic acid (3-pyridinecarboxylic acid) is is a constituent of the redox
coenzymes nicotinamide adenine dinucleotide and nicotinamide adenine
dinucleotide phosphate which are essential in energy metabolism in the
living cell. Pyridinecarboxylic
acids act as chelating agents of elements such as chromium, zinc, manganese,
copper, iron, and molybdenum in the body. They are involved in phenylalanine,
tryptophan, and alkaloids production, and for the quantitative detection of
calcium. This forms a complex with zinc, may facilitate the passage of zinc
through the gastrointestinal wall and into the circulatory system. Nicotinic
acid reacts with hemoglobin and myoglobin, which provide an application in meat
processing to form brighter colour. It acts to reduce plasma cholesterol, as a
vasodilator and to treat pellagra. It is used for the prophylaxis.
Pyridinecarboxylic acids and their derivatives can be studied for these
effects. They used as intermediate to produce pharmaceuticals and metal salts
for the application of nutritional supplements. Quinolinic acid inhibits glucose
synthesis. Fusaric acid (butyl ester of picolinic acid) inhibits dopamine
beta-hydroxylase activity.
- Nicotinic acid (Niacin;
3-Pyridinecarboxylic acid, CAS #: 59-67-6)
- Isonicotinic acid
(4-Pyridinecarboxylic acid, CAS# : 55-22-1)
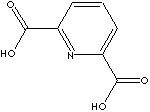
- Picolinic acid
(2-Pyridinecarboxylic acid; 98-98-6)
- Fusaric acid
(5-Butyl-2-pyridinecarboxylic acid, 536-69-6)
- Dinicotinic acid
(3,5-Pyridinedicarboxylic acid, 499-81-0)
- Dipicolinic acid
(2,6-Pyridinedicarboxylic acid, 499-83-2)
- Quinolinic acid
(2,3-Pyridinedicarboxylic acid, 89-00-9)
- Lutidinic acid
(2,4-Pyridinedicarboxylic acid, 499-80-9)
- Cinchomeronic acid
(3,4-Pyridinedicarboxylic acid, 490-11-9)
- Isocinchomeronic acid
(2,5-Pyridinedicarboxylic acid, 100-26-5)
|
|
PYRIDINE,
also called azabenzene and azine, is a heterocyclic
aromatic tertiary amine characterized by a six-membered
ring structure composed of five carbon atoms and
a nitrogen which replace one carbon-hydrogen unit
in the benzene ring (C5H5N). The simplest member of the
pyridine family is pyridine itself. It is colorless,
flammable, toxic liquid with a unpleasant odor,
miscible with water and with most organic solvents,
boils
at 115 C. Its aqueous solution is slightly alkaline. Its conjugate acid is called
pyridinium cation, C5H5NH+,
used as a oxidation agent for organic synthesis..
Pyridine is a base with
chemical properties similar to tertiary amines. Nitrogen in the ring system
has an equatorial lone pair of electrons, that does not
participate in the aromatic pi-bond. Its
aqueous solution is slightly alkaline. It is incompatible
and reactive with strong oxidizers and strong acids,
and reacts violently with chlorosulfonic acid, maleic
anhydride, oleum, perchromates, b-propiolactone,
formamide, chromium trioxide, and sulfuric acid.
Liquid pyridine easily evaporates into the air.
If it is released to the air, it may take several
months to years until it breaks down into other
compounds. Usually,
pyridine is derived from coal tar or synthesized
from
other chemicals, mainly acetaldehyde and ammonia. Pyridine compounds
are found in nature. For example, nicotine
from tobacco, ricinine from castor bean, pyridoxine or vitamin B and P products, alkaloids
(such as coniine, piperine and nicotine), and etc.
Some pyridine compounds consumed largely are;
Picoline :
Three structural isomers of methyl pyridines (alpha, beta, gamma-
positions)
Lutidine : Six structural isomers of dimethyl pyridines (2,3-,
,24-, 2,5-, 2,6-, 3,4-, 3,5- positions)
Collidine : Three structural isomers
of trimethyl pyridines (2,3,5-, 2,3,6-, 2,4,6- positions)
Pyrimidine:
Pyridine alteration containing nitrogen atoms at positions 1 and
3
Piperidine: Hexahydropyridine (saturated form)
Nicotinic acid:
pyridine-3-carboxylic acid Pyridine and
its derivatives are
very important in industrial field as well as in bio chemistry. Nucleotide consist of either a nitrogenous heterocyclic base (purine or
pyrimidine). Three major pyrimidines in living systems are cytosine, thymine, and uracil.
Pyrimidine and its derivatives are biologically important components of nucleic
acids (DNA, RNA) and coenzymes. Some pyridine system is active in the metabolism in
the body. Certain nitrogenous plant products
also have pyridine class compounds. They can be the parent compound of many drugs,
including the barbiturates. Pyridine and
its derivatives are used as solvents and starting material for the synthesis of target
compounds such as insecticides, herbicides, medicines, vitamins, food flavorings, feed additives, dyes,
rubber chemicals, explosives, disinfectants, and adhesives. Pyridine is also used as a denaturant for antifreeze mixtures, as a
dyeing assistant in textiles and in fungicides. |