PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
EXTRA NOTES
Analog of cisplatin with reduced nephrotoxicity.
Other RN: 70903-55-8
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking: http://en.wikipedia.org/
Google Scholar Search: http://scholar.google.co.kr/
http://jco.ascopubs.org/
Carboplatin (CBDCA, Bristol-Meyers, New York) is a second generation platinum analog. Preclinical and phase I clinical studies have indicated a different spectrum of toxicity compared with the parent compound. In order to study the activity of carboplatin against cancer of the head and neck, 31 patients with recurrent or metastatic disease (30 squamous-cell and one adenoid cystic carcinoma) were treated with doses of 60 to 80 mg/m2 administered daily by intravenous (IV) bolus injections for five days, repeated at every 4- to 5-week intervals. In most cases, treatment was administered on an outpatient basis. Eight patients (26%; 95% confidence interval, 12% to 45%) had complete (CR) or partial responses (PR) with a median duration of 4.5 months. Moderate bone marrow suppression was the main toxicity. Mild nausea and vomiting was unusual and no neuro- or nephrotoxicity were seen. These preliminary data suggest that carboplatin has activity against advanced squamous-cell carcinoma of the head and neck comparable with the results reported with cisplatin alone in similar patient populations. The potential advantages over the parent compound relate to the absence of nephrotoxic effects and mild gastrointestinal toxicity which allows for outpatient treatment. Because carboplatin toxicity is directly dependent on its mechanism of renal excretion, particular attention should be given for its use in patients with impaired renal function or when combined with nephrotoxic agents. Similarly, because the dose limiting toxicity with this agent is primarily hematologic, its use in combination with other myelotoxic agents should be carefully undertaken. Further studies are indicated in order to define the spectrum of activity of the new generation platinum analogs in various tumors in humans...
http://www.getwellpharma.org/
Mechanism of Action
- Carboplatin is a cisplatin analog with similar antineoplastic activity, but with a different adverse effect profile than cisplatin. Carboplatin has a carboxycyclobutane moiety replacing the chloride atoms on cisplatin. Both agents are cell cycle-nonspecific and share a similar mechanism of action.
- The major antineoplastic mechanism of action for cisplatin and carboplatin is the production of crosslinks within and between strands of deoxyribonucleic acid (DNA). Normal DNA synthesis is inhibited by this disruption of cellular DNA conformation. The dicarboxylate group on carboplatin is much more stable than the chloride groups on cisplatin, and this may possibly affect the antitumor properties of the compound. In vitro and in vivo studies indicate that the differences in cytotoxicity between cisplatin and carboplatin may be related to the kinetics of their interaction with DNA.
- Cisplatin breaks down to unstable hydrolysis products which are rapidly bound to plasma proteins and extensively filtered, with some active secretion, by the kidney, this high renal concentration is thought to be the major cause of nephrotoxicity. Carboplatin may be less nephrotoxic than cisplatin because carboplatin is more soluble and chemically stable and binds more slowly to plasma proteins. In vitro and in vivo studies indicate that the differences in cytotoxicity between cisplatin and carboplatin may be related to the kinetics of their interaction with DNA.
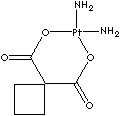
Local
Carboplatin, a platinum coordination compound which contains a platinum atom surrounded with two ammonia groups and two other ligands in a ring structure. Cisplatin is an analogue which has two chloride atoms instead of ring structure. Oxaliplatin contains a platinum atom complexed with 1,2-diaminocyclohexane and with an oxalate ligand as a leaving group. It is known that diaminocyclohexane group contributes greater cytotoxicity than cisplatin and carboplatin. Oxaliplatin is not generally cross-resistant to cisplatin or carboplatin. They are capable of forming platinum complexes producing inter- and intrastrand DNA crosslinks with neighboring guanine residues, resulting in DNA-mismatch repair (MMR) activity in the cancer cells. Carboplatin has less nephrotoxicity, neurotoxicity, ototoxicity and emetogenesis and more stability than cisplatin. They, platinum-based antineoplastics, have a broad spectrum of antitumor activity in the treatments of carcinomas of ovarian, testicular, bladder, small and non–small cell lung, head and neck carcinoma, and seminoma. They are synergistic with fluorouracil. They are radiation sensitive. The chemical designation of cisplatin is (SP-4-2)-diaminedichloroplatinum (II). It is a yellow crystalline powder; soluble in water and saline; administered intravenously. The chemical designation of carboplatin is (SP-4-2)-diammine [1,1-cyclobutanedicarboxylato (2-)-0, 0']- platinum (II). It is a white crystalline powder; soluble in water; insoluble in ethanol, acetone, and dimethylacetamide; administered intravenously. The chemical designation of oxaliplatin is SP-4-2-(1R-trans))- (1,2-cyclohexanediamine-N,N')- (ethanedioato(2-)-O,O') platinum (II). It is a white crystalline powder; soluble in water; very slightly soluble in
methanol; insoluble in ethanol, hexane, benzene and acetone.
APPEARANCE
IDENTIFICATION
Complies Test A,B
ASSAY
98.0 - 102.0% (HPLC)
PLATINUM CONTENT
52.0 - 53.0%
RELATED SUBSTANCE
0.5% max (1,1-Cyclobutane dicarboxylic acid)
WATER
0.5% max
INSOLUBLES IN WATER
0.5% max
pH
5.0 - 7.0 (10mg/ml in water)
CLARITY
Complies
SILVER
GENERAL DESCRIPTION OF ALKYLATING AGENTS AS ANTITUMOR
- Nitrogen mustard
- Chlorambucil (CAS RN: 305-03-3)
- Mechlorethamine (CAS RN: 51-75-2)
- Melphalan (CAS RN:148-82-3)
- Uracil Mustard (CAS RN: 66-75-1)
- Cyclophosphamide (CAS RN: 50-18-0)
- Iphosphamide (CAS RN: 3778-73-2)
- Nitrosoureas
- Triethylenemelamine (CAS RN: 51-18-3)
- Hexamethylmelamine (CAS RN: 645-05-6)
- Bis-chloroethylnitrosourea (CAS RN: 154-93-8)
- Chloroethylcyclohexylnitrosourea (CAS RN: 13010-47-4)
- Streptozotocin (CAS RN: 18883-66-4)
- Platinum compounds
- Carboplatin (CAS RN: 41575-94-4)
- Oxaliplatin (CAS RN: 61825-94-3)
- Cisplatin (CAS CN: 15663-27-)
- Tetraplatin (CAS RN: 62816-98-2)
- (Carboxyphthalato)platinum (CAS RN: 65296-81-3)
- Alkyl sulfonates
- Busulfan (CAS RN: 55-98-1)
- Mannosulfan (CAS RN: 7518-35-6)
- Treosulfan (CAS RN: 299-75-2)
- Aziridines
- Thiotepa (CAS RN: 52-24-4)
HAZARD OVERVIEW
Harmful if swallowed or in contact with skin. May cause an allergic skin reaction. Harmful if inhaled. May cause allergy or asthma symptoms or breathing difficulties if inhaled. May damage fertility or the unborn child. OSHA Hazards:Target Organ Effect, Toxic by inhalation., Toxic by ingestion, Harmful by skin absorption., Skin and respiratory sensitizer, Teratogen. Target Organs: Bone marrow, ears, Kidney, Nerves., Liver injury may occur., Kidney injury may occur.
GHS
Danger
PICTOGRAMS


HAZARD STATEMENTS
H302 + H312 + H332-H317-H334-H360
P STATEMENTS
P201-P261-P280-P308 + P313
![]() T
T
RISK PHRASES
46-61-20/21/22-42/43
SAFETY PHRASES
53-22-26-36/37/39-45