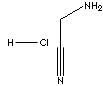
AMINOACETONITRILE HYDROCHLORIDE
PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
Glycinonitrile hydrochloride;
Glycinonitrile, monohydrochloride; alpha-Aminoacetonitrile hydrochloride; Aminoacetonitrile monohydrochloride; Aminoacetonitrilhydrochlorid (German); Aminoacetonitrilo, clorhidrato (Spanish); Aminoacétonitrile, chlorhydrate (French);
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
Stable under ordinary conditions. Moisture sensitive
GENERAL DESCRIPTION AND APPLICATIONS
·
Extraction solvent for fatty acids,
oils and unsaturated hydrocarbons
· Solvent for spinning and casting and
extractive distillation based on its selective miscibility with organic
compounds.
· Removing agent of colouring matters and aromatic
alcohols
· Non-aqueous solvent for titrations and for inorganic
salts
· Recrystallization of steroids
·
Parent compound for organic
synthesis
· Solvent or chemical intermediate in biochemistry ( pesticide
sequencing and DNA synthesis)
·
High-pressure liquid chromatographic
analysis
· Catalyst and component of transition-metal complex
catalysts
· Stabilizer for chlorinated
solvents
· Chemical intermediate and solvent for perfumes and
pharmaceuticals
Aminoacetonitrile contains both amino compound and cyano group (-C≡N), is used as an intermediate to manufacture pharmaceuticals. through the reactions of alkylation, condensation, esterification, hydrolysis, halogenation or nitration.
APPEARANCE
ASSAY
98.0% min
SULFATE
0.2% max