PRODUCT IDENTIFICATION
39831-55-5 (sulfate)
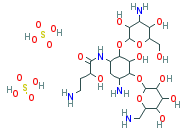
C22H43N5O13-2H2SO4
781.75
H.S. CODE
TOXICITY
O([C@H]1[C@@H](C[C@@H]([C@H]([C@@H]1O) O[C@H]1O[C @@H]([C@ @ H] (O)[C@@H]([C@H]1O)O)CN)N)N C([C@H](CCN)O)= O)[C@H] 1O[C@@H]( [C@@H](O)[C@@H]([C@H]1O)N)CO. S(O)(O)(=O) =O.S(O)(O)(=O)=O
CLASSIFICATION
Antibacterial, Antibiotic
EXTRA NOTES
Other RN: 37357-52-1
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking - Amikacin
Google Scholar Search - Amikacin sulfate
Drug Information Portal (U.S. National Library of Medicine) - Amikacin sulfate
PubChem Compound Summary - Amikacin sulfate
Drug Bank - Amikacin
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Amikacin sulfate
http://www.ebi.ac.uk/ - Amikacin
http://www.ncbi.nlm.nih.gov/ - Amikacin sulfate
Local:
Amikacin sulfate [ chemically : D-Streptamine, O-3-amino-3-deoxy-alpha-D- glucopyranosyl- (1-6)-O-(6-amino-6-deoxy-alpha-D-glucopyranosyl-(1-4))- N1-(4-amino-2-hydroxy-1- oxobutyl)- 2-deoxy-, (S)-sulfate (1:2) (salt)] is a semi-synthetic aminoglycoside antibiotic derived from kanamycin. Its spectrum is somewhat broader than that of other aminoglycosides, including Serratia and Acinetobacter species, as well as certain staphylococci and streptococci. It is the most effective agent against gram-negative bacilli that have some aminoglycoside resistance. It is used for the treatment of serious infections resistant to gentamicin and tobramycin (bone infections, respiratory tract infections, endocarditis, and septicemia).
Aminoglycoside is any of a group of antibiotics derived from various species of Streptomyces or Micromonosporaceae, while some of which are produced synthetically. Aminoglycosides inhibit bacterial protein synthesis by binding with the 30S ribosomal subunit and in some cases the 50S subunit, causing miscoding and thus ihibiting initiation and elongation during protein synthesis. They include;
- Amikacin
- Gentamicin
- Kanamycin
- Neomycin
- Netilmicin
- Paromomycin
- Streptomycin
- Tobramycin
APPEARANCE
674 - 790 ��m/mg (Anhydrous basis)
IDENTIFICATION
pass (A,B,C,D,E)
SULFATE
3.0% max
pH
CLARITY OF SOLUTION
pass
LOSS ON DRYING
13.0% max
OPTICAL ROTATION
+76.0 ~ +84.0°
CLARITY OF SOLUTION
90.0% min
RESIDUE ON IGNITION
1.0% max
HAZARD OVERVIEW
OSHA Hazards:Target Organ Effect, Teratogen. Target Organs:ears, Kidney. May be harmful if inhaled. May cause respiratory tract irritation. May be harmful if absorbed through skin. May cause skin irritation. May cause eye irritation. May be harmful if swallowed.
GHS
Warning
PICTOGRAMS
HAZARD STATEMENTS
H303
P STATEMENTS