PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
SOLVENT SOLUBILITY
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions
GENERAL DESCRIPTION AND APPLICATIONS
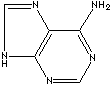
- Adenine: a pyrimidine base
- Adenosine Triphosphate (ATP) : a nucleotide composed of adenine, the sugar ribose, and three phosphate groups; involved in energy metabolism and required for RNA synthesis. It exists in cells in a form of high-energy phosphate bond to store and transport chemical energy. The pyrophosphate nature of the bonds between ATP's three phosphate radicals results in a powerful donor of phosphate groups to suitable acceptors. When it is broken down by hydrolysis, it yields ADP (adenosine diphosphate), inorganic phosphorus, and energy. The free energy derived from hydrolysis of ATP is used to drive metabolic reactions including the synthesis of nucleic acids and proteins, to move molecules against concentration gradients (active transport), and to produce mechanical motion (contraction of microfibrils and microtubules). ADP can be further broken down to yield adenosine monophosphate (AMP), additional phosphorus, and more energy. When the phosphorus and energy are immediately used to drive other reactions, such as the synthesis of UDP (uridine diphosphate), an RNA precursor, from UMP (uridine monophosphate), the pair of reactions are said to be coupled. New ATP is produced from AMP using the energy released from the breakdown of fuel molecules, such as fat and glucose which is broken down into pyruvate in the cytosol. Two molecules of ATP are generated for each molecule of glucose. ADT can be converted back to ATP by the processes of oxidative phosphorylation and substrate-level phosphorylation.
- Adenosine Diphosphate (ADP) : a nucleotide composed of pyrophosphate of adenosine, involved in energy metabolism; it is produced by hydrolysis of ATP and converted back to ATP by the processes of oxidative phosphorylation and substrate-level phosphorylation.
- Adenosine Monophosphate (AMP, Adenylic acid.) : a nucleotide, 5'-phosphate of adenosine, produced by the hydrolysis of ATP and converted to ADP by adenylate kinase. Involved in the reactions of intracellular energy transfers.
- Cyclic Adenosine Monophosphate (cAMP) : cyclic AMP containing an additional ester linkage between the phosphate and ribose units; serves as an intracellular and, in some cases, extracellular secondary messenger mediating the action of many peptide or amine hormones. It also plays a role in the transcription of some genes.
- Deoxyadenosine (dA) : a purine nucleoside composed of adenine linked by its N9 nitrogen to the C1 carbon of deoxyribose. (deoxy-, also called desoxy, is a prefix for the designation of compounds which contain one less atom of oxygen than the reference substance).
- Deoxyadenosine diphosphate (dADP) : a nucleotide, 5'-pyrophosphate of deoxyadenosine.
- Deoxyadenosine monophosphate (dAMP) : a nucleotide, 5'-phosphate of deoxyadenosine, occurring in deoxyribonucleic acid.
- Deoxyadenosine triphosphate (dATP): a nucleotide, the 5'-triphosphate of deoxyadenosine; activated precursor in DNA synthesis.
Chemically modified nucleotides substituted or attached by special chemical groups or elements are studied and used to inactivate the normal biological operation in the living organism and the function of important enzymes.
GENERAL DESCRIPTION OF PURINE: Purine is a heterocyclic compound featured by a fused pyrimidine and imidazole rings composed of carbon and nitrogen atoms. The simplest one is purine itself and the two major purines are adenine(6-Aminopurine) and guanine(2-Amino-6-hydroxypurine) which are two bases components of nucleic acid and the nucleotides. Purine itself is not found in nature, but as substituted purines such as methyled, hydroxyl and amino substituted. In addition to adenine and guanine, a group of chemical compounds called purine base include hypoxanthine (6-oxypurine), xanthine (2,6-dioxypurine), uric acid (2,6,8-trioxypurine), and theobromine (3,7-dimethyl xanthine). Theophylline and caffeine are a member of methylated purine family. Purines are biologically important in In medicine and biological research.Wikipedia Linking: http://en.wikipedia.org/wiki/Adenine
http://www.chem.duke.edu/
Adenine is a purine. Purines are six-membered rings attached to five membered
rings. When Adenine is attached to DNA, it forms a bond with another molecule
called Thymine, a pyrimidine, on the other side of the DNA strand. It is these
bonds which give DNA its double-helix structure. The sequence of DNA, or the
order in which nucleotides are placed, allows for the diversity among all living
organisms. The importance of Adenine to RNA is similar to that of DNA. Besides DNA and RNA, Adenine is also an important part of adenosine
triphosphate, or ATP. Adenosine triphosphate is the nitrogenous base adenine
bonded to a five carbon sugar. This molecule is important because it has the
ability to phosphorylize, or add a phosphate group to, other molecules. This
transfer of a phosphate group allows energy to be released. It is this energy
which is used by cells in living organisms. This is why the molecules ATP, and
its nitrogenous base Adenine, are so important.
http://www.encyclo.co.uk/
Look up: Adenine
http://nationaldiagnostics.com/
Biological Macromolecules
ADENINE BASE
APPEARANCE
ASSAY
HEAVY METALS
10ppm max
RESIDUE ON IGNITION
0.1% max
0.5% max
ADENINE HCl
APPEARANCE
ASSAY
HEAVY METALS
10ppm max
RESIDUE ON IGNITION
0.1% max
5.0% max
ADENINE SULFATE
APPEARANCE
ASSAY
HEAVY METALS
10ppm max
RESIDUE ON IGNITION
0.1% max
9.0% max