PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions. Moisture Sensitive.
APPLICATIONS
Imidazole is a heterocyclic compound of five-membered diunsaturated ring
structure composed of three carbon atoms and two nitrogen atoms at nonadjacent
positions. The simplest member of the imidazole family is imidazole itself,
colorless to pale yellow crystalline solid with a weak aminelike odor; soluble
in water and alcohol, melts at 89 C, boils at 256 C. Imidazoles are poorly
soluble in water generally, but are dissolved in organic solvents, such as
chloroform, propylene glycol, and polyethoxylated castor oil. Imidazole ring is
found in histidine (an essential amino acid) and histamine, the decarboxylated
compound from histamine. Some imidazole compounds inhibit the biosynthesis of
ergosterol, required in cell membrane in fungal. They have antibacterial,
antifungal, antiprotozoal, and anthelmintic activity. Several distinct
phenylimidazoles are therapeutically useful antifungal agents against either
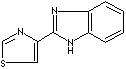
superficial or systemic infections. Thiabendazoles which have anthelmintic and
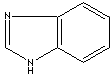
antifungal properties are imidazole class compounds. Benzimidazole is a dicyclic
compound having midazole ring fused to benzene. Benzimidazole structure is a
part of the nucleotide portion of vitamin B12 and the nucleus in some drugs such
as proton pump inhibitors and anthelmintic agents. Imidazole has two nitrogen
atoms.
The one is slightly acidic, while the other is basic. Imidazole and its
derivatives are widely used as intermediates in synthesis of organic target
compounds including pharmaceuticals, agrochemicals, dyes, photographic
chemicals, corrosion inhibitors, epoxy curing agents, adhesives and plastic
modifiers. Some imidazole analogues which contain nitrogen in five-membered ring
structure are:
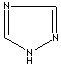
- Triazole: An analog of imidazole.It has three nitrogen atoms and two carbon atoms at nonadjacent positions in the ring system.
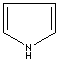
- Pyrrole: An analog of imidazole. It has only one nitrogen atom in the ring system. Pyrrole ring system is involved in coloured products (green pigment, chlorophyll; red, hemoglobin; , blue, indigo) in nature.
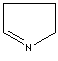
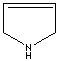
- Pyrroline: A pyrrole in which one of the two double bonds are hydrogenated.
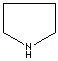
- Pyrrolidine: The saturated tetrahydropyrrole, a part of the structures of amino acids (proline, hydroxyproline and hygrine).
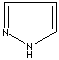
- Pyrazole: 1,2-Diazole (Imidazole isomer). The nitrogen positions are 1 and 2. It is not found in nature
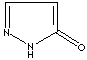
- Pyrazolone: Pyrazole analog with ketone group at 5 positon
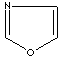
- Oxazole: an analog of imidazole. The nitrogen atom in position 1 is replaced by oxygen.
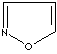
- Isoxazole: an analog of pyrazole. The nitrogen atom at position 1 is replaced by oxygen.
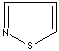
- Isothiazole:an analog of pyrazole. The nitrogen atom at position 1 is replaced by sulfur.
|
|
|
|
|
|
1,2,4-Triazole |
Pyrrole |
1-Pyrroline |
3-Pyrroline |
|
|
|
|
|
|
Pyrrolidine |
Pyrazole |
Pyrazolone |
Oxazole |
|
|
|
|
|
|
Isoxazole |
Isothiazole |
Benzimidazole |
Thiabendazole |
APPEARANCE
ASSAY
98.0% min
25kgs
in fiber drum