PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
c1ccc2c(c1)c(cc(=O)o2)O
CLASSIFICATION
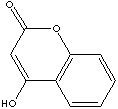
Coumarin
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
210 - 215 C (Decomposes)
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
USA.gov - 4-Hydroxycoumarin
Wikipedia Linking - 4-Hydroxycoumarin
Google Scholar Search - 4-Hydroxycoumarin
U.S. National Library of Medicine - 4-Hydroxycoumarin
PubChem Compound Summary - 4-Hydroxycoumarin
KEGG (Kyoto Encyclopedia of Genes and Genomes) - 4-Hydroxycoumarin
http://www.ebi.ac.uk/chebi/ - 4-Hydroxycoumarin
http://www.ncbi.nlm.nih.gov/ - 4-Hydroxycoumarin
EPA - Substance Registry Services - 4-Hydroxycoumarin
Local:
Coumarin (anhydride of o-coumaric acid) is white, crystalline lactone,
obtainable naturally from several plants, such as tonka bean, lavender, sweet
clover grass, strawberries, and cinnamon, or produced synthetically from an
amino acid, phenylalanine. It is the principle of dicumarol which inhibits the
hepatic synthesis of the vitamin K–dependent coagulation factors. Coumarin
derivatives are used widely as anticoagulants (such as
warfarin, -OH group is attached at 4 position) for the treatment of disorders in which there is excessive or
undesirable clotting, such as thrombophlebitis, pulmonary embolism, and certain
cardiac conditions. Coumarin derivatives are also used as rodenticides due
to the property of causing fatal hemorrhaging. Coumarin
has the characteristic odour like that of vanilla beans. It is used for the
preparation of perfumes, soaps, flavourings. The coumarin nucleus (benzo-2-pyrone) is derived cinnamic acid (phenylacrylic
skeleton) in the biosynthesis. Accordingly, the hydroxy group attached to
coumarin structure at 7 position is important in biosynthesis pathway.
Umbelliferone (7-hydroxy coumarin), esculetin (6,7-Dihydroxycoumarin),
scopoletin (7-hydroxy-6-methoxycoumarin) are the widespread coumarins in nature.
Synthetic 7-hydroxy coumarins are used to absorb ultraviolet rays in sunscreen
cosmetics and used in the synthesis of drugs especially anticancer.
APPEARANCE
98.0% min
210 - 215 C
WATER
0.5% max
HAZARD OVERVIEW
GHS (Globally Harmonised System) Classification: Acute toxicity (Oral). Skin irritation. Eye irritation. Specific target organ toxicity - single exposure. Hazard statements: Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation.
GHS
Warning
PICTOGRAMS

HAZARD STATEMENTS
H302-H315-H319-H335
P STATEMENTS
P261-P305 + P351 + P338
![]()
RISK PHRASES
22-36/37/38
SAFETY PHRASES
26-36