PRODUCT IDENTIFICATION
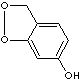
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
SOLVENT SOLUBILITY
REFRACTIVE INDEX
Stable under ordinary conditions
GENERAL DESCRIPTION APPLICATIONS
Sesamol, which is derived from sesame seed lignans, is reportedly an antioxidant. Nitric oxide (NO), the most important vascular relaxing factor, is regulated in the endothelium. In addition, NO is involved in protecting endothelium and has antiatherosclerotic and antithrombotic activities. The endothelium produces NO through the regulation of both endothelial NO synthase (eNOS) expression and activity in endothelial cells. This study sought to investigate the effect of sesamol on NO released from human umbilical vein endothelial cells (HUVEC) and to examine the expression and activity of eNOS. Sesamol induced NO release from endothelial cells in a dose-dependent manner (from 1 to 10 microM), as measured 24 h after treatment; the expression of the eNOS gene at both transcription and translation levels; and NOS activity in endothelial cells. The content of cGMP was also increased by sesamol through NO signaling. The transcription of eNOS induced by sesamol was confirmed through the activation of PI-3 kinase-Akt (protein kinase B) signaling. The results demonstrate that sesamol induces NOS signaling pathways in HUVEC and suggest a role for sesamol in cardiovascular reactivity in vivo.(http://www.ncbi.nlm.nih.gov/)
Sesamin and sesamolin, with two methylenedioxy bridges have potentially 4 functional OH groups, and scavenged ROS in cell-free condition better than sesamol with 2 OH groups. This suggests that the higher inhibition of LPS-induced NO production by sesamin and sesamolin may be due to the difference in numbers of functional OH group. A watersoluble form of sesamol, 1,2,4-benzenetriol, has a similar potency in inhibiting LPS-induced NO production (our unpublished observation). The methylenedioxy bridge of sesamin and sesamolin, is formed by a redox reaction of cytochrome P450 in the presence of O2 and NADPH. Sesamin is metabolized in the liver and converted to an antioxidative form that inhibits superoxide production in aortic endothelium. The metabolized forms of sesamin show stronger scavenging activity than sesamin against ROS in O2- ,.OH, 2,2-diphenyl-1-picrylhydrazyl radical (DPPH.) and TBARS assay. However, the free radical scavenging power of sesame crude extract by the DPPH. assay shows the order: sesamin> sesamolin> sesaminol triglucoside>sesaminol diglucoside. Using DPPH. kinetic model to study the free radical scavenging capacity, the second-order rate constant k2 values of the sesame antioxidants are calculated for the quenching reaction with DPPH. radical. The k2 values are in the order sesamol>sesamol dimmer>sesamin> sesamolin> sesaminol triglucoside>sesaminol diglucoside (http://www.bentham.org/)
Sesamol, can be naturally obtained from sesame seed (Sesamum indicum L.) has been reported to have antioxidant property which protect the body from damage from free radicals. It is a white crystalline solid sparingly soluble in water, but miscible with most oils. It also may inhibit the growth of fungi and yeasts in oils. Sesamol derivatives prepared with nicotinic acid and clofibric acid are expected to have synergistic hypolipidemic agents. Sesamol is used in the synthesis of anticancer drugs and antidepressants (Paroxetine).
APPEARANCE
ASSAY
98.0% min
LOSS ON DRYING
0.5% max
HEAVY METAL
20ppm max
5kgs in can