PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
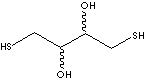
C([C@@H]([C@@H](CS)O)O)S
CLASSIFICATION
Disulfide bridge cleavage reagent, Reducing reagent
EXTRA NOTES
Other RN: 27565-41-9; 28823-08-7; 1377983-58-8; 214119-27-4
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions. Hygroscopic.
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking - Dithiothreitol
Google Scholar Search - Dithiothreitol
Drug Information Portal (U.S. National Library of Medicine) - Dithiothreitol
PubChem Compound Summary - Dithiothreitol
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Dithiothreitol
Chemical Entities of Biological Interest (ChEBI) - Dithiothreitol
http://www.ncbi.nlm.nih.gov/ - Dithiothreitol
http://www.sigmaaldrich.com/
An excellent reagent for maintaining SH groups in reduced state; quantitatively reduces disulfides. DTT is effective in sample buffers for reducing protein disulfide bonds prior to SDS-PAGE. DTT can also be used for reducing the disulfide bridge of the cross-linker N,N′-bis(acryloyl)cystamine to break apart the matrix of a polyacrylamide gel. DTT is less pungent and is less toxic than 2-mercaptoethanol. Typically, a seven fold lower concentration of DTT (100 mM) is needed than is used for 2-mercaptoethanol (5% v/v, 700 mM).
Local
Erythritol is a a four-carbon sugar alcohol formed from erythrose by reduction
of the carbonyl group; found in algae, lichens, grasses, and certain fungi. It
is it is about twice as sweet as sucrose and used as a sweetener or food
additive. But it is not absorbed by the digestive system in the body. sugar
alcohol which has been approved for use in the US as a food additive and
sweetener. Erythritol (more correctly meso-erythritol; meso- means inactive) is
the optically inactive isomeric tetritol among three stereoisomers. The other
two stereoisomeric tetritols are D- and L-threitol. The only existing
three-carbon sugar alcohol (triitol) is glycerol. The four stereoisomeric
pentitols are xylitol, ribitol, and D- or L-arabitol. Mercaptan esters of
threitol, low molecular weight thiols, reduces disulfide bonds quantitatively
and maintains monothiols in the reduced state, which provides the applications
including protein processing, induction of genes, oligonucleotide synthesis.
Cleland's reagent is the commonest reagent for maintaining -SH groups in reduced
state. Mercaptan esters of threitol have been studied as anti-cancer agents by
acting as alkylating agents to damage the cancer cell DNA.
APPEARANCE
99.0% min
HEAVY METALS
MOISTURE
0.1% max
HAZARD OVERVIEW
GHS
Warning
PICTOGRAMS

HAZARD STATEMENTS
H302-H315-H319-H335
P STATEMENTS
P261-P305 + P351 + P338
![]()
RISK PHRASES
22-36/37/38
SAFETY PHRASES
26-36