PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
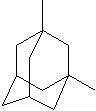
Adamantane
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
201 C
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
51 C
Stable under ordinary conditions
EXTERNAL LINKS & GENERAL DESCRIPTION
PubChem Compound Summary - 1,3-Dimethyladamantane
KEGG (Kyoto Encyclopedia of Genes and Genomes) - 1,3-Dimethyladamantane
http://www.ebi.ac.uk/chebi/ - 1,3-Dimethyladamantane
http://pubs.acs.org/
New
Synthetic Approach to Memantine Hydrochloride starting from 1,3-Dimethyl-adamantane
Local:
Adamantane consists of four cyclohexanes fused each other in chair
conformations. It is the most stable isomer of formula, C10H16; one of the
highest melting point hydrocarbons (> 210 C); boiling point cannot be determined
directly as it sublimes easily. Its stability is from strain free and highly
symmetrical structure (all C-C bonds are set in and all Cs are tetrahedral). Its systematical name is tricyclo[3.3.1.1(3,7)]decane which
means bicyclo[3.3.1]decane with an extra one-carbon bridge between C3 and C7. An
alkyl substituent is axial in one ring and equatorial in another. Polymantanes
have the same structure as the diamond lattice featuring highly symmetrical and
strain free and are named as diamondoids . The general molecular formula of homologous diamondoid is
C4n+6H4n+12.
Diamondoids have one or more cages and are called diamantane, triamantane, and polymantanes.
There are numerous structural isomers as well. Aadamantane itself
is the simplest
diamondoid. Some examples are; iceane (C12H18),
diadamantane (C14H20),
triadamantane (C18H24),
isotetramantane (C22H28),
cyclohexamantane (C26H30),
superadamantane
(C35H36).
Natural diamondoids are extracted and purified from petroleum deposits.
Diamondoids are being investigated in the new field of
nanotechnoloy. Clinically, amantane and its derivatives are employed chiefly for the synthesis of
antiviral drugs as well as neurological drugs such as rimantadine
and Parkinson’s disease drugs. Their unique structures have the possibility to
be applied to produce a broad range of biologically and pharmaceutically active
drugs such as anticancer and anti-microbial agents. An application of 1,3-Dimethyladamantane
can be found in the synthesis of
the drug called memantine used in protecting the brain
nerve cells.
APPEARANCE
ASSAY
99.0% min
WATER
0.5% max
DENSITY
0.865 - 0.905
Hazard Overview
Combustible Liquid
GHS
PICTOGRAMS

HAZARD STATEMENTS
H226 Flammable liquid and vapour
RISK PHRASES
10 Flammable
SAFETY PHRASES
16 Keep away from sources of ignition - No smoking