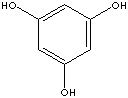
1,3,5-TRIHYDROXYBENZENE
PRODUCT IDENTIFICATION
H.S. CODE
2907.29
TOXICITY
Phloroglucinol; s-Trihydroxybenzene; Benzene-s-triol;
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
SOLVENT SOLUBILITY
4.3
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
GENERAL DESCRIPTION & EXTERNAL LINKS
Members of phloroglucinol molecules
|
Product |
CAS RN |
| 2,4,6-Trihydroxybenzoic acid | 83-30-7 |
| 2-Methylphloroglucinol | 88-03-9 |
| Phloroglucinol | 108-73-6 |
| 1-(2,4,6-Trihydroxyphenyl)ethanone | 480-66-0 |
| 2,4,6-Trihydroxybenzaldehyde | 487-70-7 |
| Phloroglucinol dimethyl ether | 500-99-2 |
| 1,3,5-Trimethoxybenzene | 621-23-8 |
| 2,4-Diacetylphloroglucilmethane | 726-28-3 |
| 2-Bromo-1,3,5-trimethoxybenzene | 1131-40-4 |
| 2,4-Diacetylphloroglucinol | 2161-86-6 |
| Flopropione | 2295-58-1 |
| 1,3,5-Benzenetriol triacetate | 2999-40-8 |
| 2,4,6-Tribromo-1,3,5-benzenetriol | 3354-82-3 |
| 2,4-Dimethylphloroglucinol | 4463-02-9 |
| Trimethylphloroglucinol | 4463-03-0 |
| 1,3,5-Benzenetriol dihydrate | 6099-90-7 |
| Polyphloroglucinol phosphate | 9014-68-0 |
| 2-Nitrophloroglucinol | 16600-92-3 |
| 1,3,5-Trimethoxy-2-(phenylmethyl)benzene | 22807-99-4 |
| 2,4-Dihydroxy-6-(3,4-dihydroxybenzoyloxy)benzoic acid | 30048-34-1 |
| Trinitrophloroglucinol lead salt | 51325-28-1 |
| Trichlorophloroglucinol | 56961-23-0 |
| 2-Chloro-1,3,5-trimethoxybenzene | 67827-56-9 |
| 2-Ethyl-1,3,5-trimethoxybenzene | 67827-55-8 |
| 2-(3-(3-Hydroxy-4-methoxyphenyl)propyl)-1,3,5-benzenetriol | 70413-04-6 |
| Spasfon | 79173-14-1 |
| Vidalol B | 137182-40-2 |
| Vidalol A | 137182-39-9 |
Wikipedia Linking: http://en.wikipedia.org/wiki/Phloroglucinol
Phloroglucinol compounds of therapeutic interest: global patent and technology status - Phloroglucinol compounds, both synthetic as well as natural, have shown a vast array of biological activities. There are a wide range of applications of phloroglucinol compounds in pharmaceuticals, cosmetics, textiles, paints and dyeing industries. Although many of the phloroglucinols have shown promising results in various biological assays, very few have reached clinics. Objective: To compile the patented information on various therapeutically active phloroglucinol molecules, so that technologies used in isolation and activity assessment of these compounds could be unearthed and the compiled information be utilized for further development of these molecules. Methods: The European Patent Office database (official website: espacenet.com) was searched with a keyword ��phloroglucinol��. In addition, patents were searched using names of compounds listed in our previous review. Conclusions: This class holds potential for development of molecules in various therapeutic areas. There exist a number of patents on preparations that have phloroglucinol compounds as active ingredient(s). Many such preparations have been tested in vitro and/or in vivo for their efficacy and proven to be active and non-toxic. Commercialization of existing technology on phloroglucinol molecules can yield fruitful results......... (http://www.anu.edu.au/)
APPEARANCE
CONTENT
99.0%
MOISTURE
0.5% max