PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
LD50 orl-rat 1000-2000 mg/kg
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
260 C (Decomposes)
AUTOIGNITION
490 C
NFPA RATINGS
REFRACTIVE INDEX
170 C
Stable under ordinary conditions
GENERAL DESCRIPTION & EXTERNAL LINKS
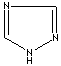
Triazole is one of a class of organic heterocyclic compounds containing a five-membered diunsaturated ring structure composed of three nitrogen atoms and two carbon atoms at nonadjacent positions. The simplest member of the Triazole family is triazole itself, white to pale yellow crystalline solids with a weak characteristic odor; soluble in water and alcohol, melts at 120 C, boils at 260 C. Triazole and its derivatives are used for biological activities such as antiviral, antibacterial, antifungal and antituberculous. 1,2,4-Triazole is used as an intermediate for phytosanitary, pharmaceutical, medicine and pesticide products, photoconductor, and copying systems
Wikipedia Linking: http://en.wikipedia.org/wiki/Triazole
Preparation of a nicotinamide nucleoside analog via enzymatic ribosidation of 1,2,4-triazole, Annulating agents for construction of bicyclic 1,2,4-triazole systems, Efficient synthesis of 3-hydroxymethyl-4-phenyl-1,2,4-triazole, Synthesis of selectively C-3 and N-4 substituted [1,2,4]-triazoles, Attempted ring addition to 3,5-dimethyl-1,2,4-triazole, Anions of 1-substituted-1,2,4-triazoles as nucleophiles, Annulation onto 1,2,4-triazole via a Michael-type reaction, Bicyclic ring systems from appropriately substituted triazoles, Solvent and counterion effects on the NMR of disubstituted triazoliums......... ( http://www.wellesley.edu/)
AN EASY AND DIRECT METHOD FOR THE SYNTHESIS OF 1,2,4-TRIAZOLE DERIVATIVES THROUGH CARBOXYLIC ACIDS AND HYDRAZINOPHTHALAZINE (http://quimicanova.sbq.org.br/)
APPEARANCE
White to Yellowish Crystal
CONTENT
95.0% min
MELTING POINT
117 C min
EXAMPLES OF AZOLE FUNGICIDE
|
IMIDAZOLE |
TRIAZOLE |
||
|
FUNGICIDE |
CAS RN |
FUNGICIDE |
CAS RN |
|
Climbazole |
38083-17-9 |
Amisulbrom |
348635-87-0 |
|
Clotrimazole |
23593-75-1 |
Azaconazole |
60207-31-0 |
|
Cyazofamid |
120116-88-3 |
Bitertanol |
55179-31-2 |
|
Fenamidone |
161326-34-7 |
Bromuconazole |
116255-48-2 |
|
Fenapanil |
61019-78-1 |
Cyproconazole |
94361-06-5 |
|
Glyodin |
556-22-9 |
Diclobutrazol |
75736-33-3 |
|
Imazalil |
35554-44-0 |
Difenoconazole |
119446-68-3 |
|
Isovaledione |
|
Diniconazole |
83657-24-3 |
|
Oxpoconazole |
134074-64-9 |
Diniconazole-M |
83657-18-5 |
|
Pefurazoate |
101903-30-4 |
Epoxiconazole |
135319-73-2
or |
|
Triazoxide |
72459-58-6 |
Etaconazole |
60207-93-4 |
|
Triflumizole |
99387-89-0 |
Fenbuconazole |
114369-43-6 |
|
|
|
Fluotrimazole |
31251-03-3 |
|
|
|
Fluquinconazole |
136426-54-5 |
|
|
|
Flusilazole |
85509-19-9 |
|
|
|
Flutriafol |
76674-21-0 |
|
|
|
Furconazole |
112839-33-5 |
|
|
|
Furconazole (cis) |
112839-32-4 |
|
|
|
Hexaconazole |
79983-71-4 |
|
|
|
Ipconazole |
125225-28-7 |
|
|
|
Metconazole |
125116-23-6 |
|
|
|
Myclobutanil |
66246-88-6 |
|
|
|
Penconazole |
66246-88-6 |
|
|
|
Propiconazole |
60207-90-1 |
|
|
|
Prothioconazole |
178928-70-6 |
|
|
|
Quinconazole |
103970-75-8 |
|
|
|
Simeconazole |
149508-90-7 |
|
|
|
Tebuconazole |
107534-96-3 |
|
|
|
Tetraconazole |
112281-77-3 |
|
|
|
Triadimefon |
43121-43-3 |
|
|
|
Triadimenol |
55219-65-3 |
|
|
|
Triazbutil |
16227-10-4 |
|
|
|
Triticonazole |
131983-72-7 |
|
|
|
Uniconazole |
83657-22-1 |
|
|
|
Uniconazole-P |
83657-17-4 |