ZEARALENONE
PRODUCT IDENTIFICATION
17924-92-4
241-864-0
H.S. CODE
TOXICITY
Zearalenone; Zenone; trans-Zearalenone;
(-)-Zearalenone; (10S)-Zearalenone; (S)-(-)-Zearalenone; (S)-Zearalenone; 6-(10-Hydroxy-6-oxo-trans-1-undecenyl)-beta-resorcylic acid lactone; trans-14,16-Dihydroxy-3-methyl-7-oxobenzoxacyclotetradec-11-en-1-one; trans-6-(10-Hydroxy-6-oxo-1-undecenyl)resorcylic acid mu-lactone; Other RN: 18695-28-8
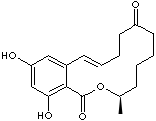
c12c(cc(cc2O)O)C=CCCCC(=O)CCC[C@@H](OC1=O)C
CLASSIFICATION
Non-steroidal estrogen
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
Slightly soluble in hexane. Soluble in benzene, acetonitrile, dichloromethane, methanol, acetone.
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Zearalenone
PubChem Compound Summary - Zearalenone
IPCS INCHEM - Zearalenone
Drug Bank - Zearalenone
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Zearalenone
http://www.ebi.ac.uk/ - Zearalenone
http://www.ncbi.nlm.nih.gov/ - Zearalenone
http://www.sigmaaldrich.com/Biochem/physiol Actions: Zearalenone, a fungal mycotoxin produced by Fusarium, binds the estrogen receptor (ER) and is uterotropic in the newborn rat. It is a common contaminant in cereal grain used for animal and human food, and exerts an estrogenic activity that modulates/disrupts endocrine function in animals and possibly humans.
http://www.ansci.cornell.edu/
Mycotoxins
produced by Fusarium spp. are of two general types: 1) the nonestrogenic
trichothecenes, including DON, nivalenol, T-2 toxin, and diacetoxyscripenol,
and 2) the mycoestrogens, including Zearalenone (ZEN) and zearalenol
. Zearalenone and zearalenol are both estrogenic resorcylic acid
lactone compounds produced by the fungi Fusarium spp. (Diekman and
Green, 1992) Despite their structural dissimilarity to the steriodal estrogens,
ZEN and several of its derivatives possess estrogenic activity.
ZEN undergoes a folding such that hydroxyl or potential hydroxyl
groups become appropriately orientated to facilitate binding to
tissue receptors that normally bind estrogens. Similar binding affinities
for ZEN have been determined for the estrogen receptor in sheep
and calf uterus.(Diekman and Green, 1992)
Local:
Zeranol is a non-steroidal estrogenic growth stimulant that is used in
veterinary medicine and has been used for human. The use for growth promotion in
food animals was banned in the EU. An anabolic-estrogenic agent promote cell
growth and division, resulting in growth of muscle tissue and sometimes bone
size and strength. Testosterone and androgen are examples. Zeranol is also
called zearalenol as it is a reduction product of zearalenone, an estrogenic
mycotoxin product. Oestradiol-17beta, testosterone, progesterone, and trebolne
are also used as growth stimulants for food animals.
Non-steroidal estrogen analog |
CAS RN |
| Equol | 531-95-3 |
| 2-sec-Butylphenol | 89-72-5 |
| Diethylstilbestrol | 56-53-1 |
| Bisphenol A | 80-05-7 |
| Dienestrol | 84-17-3 |
| Coumestrol | 479-13-0 |
| Allenolic acid | 553-39-9 |
| Chlorotrianisene | 569-57-3 |
| 4-Octylphenol | 1806-26-4 |
| Hexestrol | 5635-50-7 |
| Zearalenone | 17924-92-4 |
| Zeranol | 26538-44-3 |
APPEARANCE
98.0% min
MELTING POINT
159 ~ 162 C
HAZARD OVERVIEW
OSHA Hazards:Target Organ Effect, Irritant, Teratogen, Reproductive hazard. Target Organs: Reproductive system.
GHS
PICTOGRAMS


HAZARD STATEMENTS
H314-H361
P STATEMENTS
P280-P305 + P351 + P338-P310
![]()
RISK PHRASES
34
SAFETY PHRASES
26-36/37/39-45