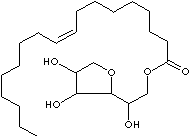
| SORBITAN
OLEATE
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PRODUCT IDENTIFICATION |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS NO. | 1338-43-8 |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EINECS NO. | 215-665-4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FORMULA | C24H44O6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MOL WT. | 428.61 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
H.S. CODE |
3402.13 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
TOXICITY |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SYNONYMS | SPAN 80; Sorbitan Monooleate; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sorbitan oleate; Monodehydrosorbitol monooleate; Sorbitan monooleic acid ester; Sorbitan, mono-9-octadecenoate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES |
Sorbitol, Fatty Acid |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CLASSIFICATION |
SURFACTANTS / |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PHYSICAL STATE | yellow to brown liquid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MELTING POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BOILING POINT |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SPECIFIC GRAVITY |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SOLUBILITY IN WATER | Insoluble | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| pH | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VAPOR DENSITY |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HLB VALUE | 4.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AUTOIGNITION |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NFPA RATINGS |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
REFRACTIVE INDEX |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FLASH POINT | > 140 C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| STABILITY |
Stable under ordinary conditions |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
GENERAL DESCRIPTION & APPLICATIONS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sorbitol is a white, sweetish, hygroscopic, crystalline sugar alcohol of
six-carbon. It is found naturally in various berries and fruits. Or it is
prepared synthetically by high-pressure catalytic hydrogenation of glucose sugar
derived from cornstarch. It melts at 93 to 98 C depending on the form. It is
used as a a sweetening agent, food additive, toothpaste, tobacco, toiletries and
in cosmetics. It is used for vitamin-C fermentation. It is used as a excipient
and intravenous osmotic diuretic in pharmaceutical fields. It is also used in
the manufacture of polyethers for polyurethanes and surfactants. The term
sorbitan describes the anhydride form of sorbitol, whose fatty acids are
lipophilic whereas sorbitol body is hydrophilic. This bifunctionality in one
molecule provides the basic properties useful in cleaners, detergents, polymer
additives, and textile industry as emulsifiers, wetting agents, and viscosity
modifiers. Sorbitan esters are rather lipophilic (or hydrophobic) surfactants
exhibiting low HLB (Hydrophilic-Lipophilic Balance) values; having an affinity
for, tending to combine with, or capable of dissolving in lipids (or
water-insoluble). While, the ethoxylated sorbitan esters are hydrophilics
exhibiting high HLB values; having an affinity for water; readily absorbing or
dissolving in water. The type of fatty acid and the mole number of ethylene
oxide provides diverse HLB values for proper applications.
HLB numbers describe following characterestics: <10 : Lipid soluble (or
water-insoluble) HLB values of sorbitan compounds are:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SALES SPECIFICATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
APPEARANCE |
yellow to brown liquid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACID VALUE |
7.0
max
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HYDROXYL VALUE |
190
- 240
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAP VALUE |
135 - 160 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MOISTURE |
1.0%
max
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TRANSPORTATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PACKING |
180kgs in drum |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HAZARD CLASS | Not regulated | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UN NO. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
GENERAL DESCRIPTION OF FATTY ACID |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fatty Acids are aliphatic carboxylic acid with varying length hydrocarbon chains at one end of the chain joined to terminal carboxyl (-COOH) group at the other end. The general formula is CnH2n+1COOH or R-(CH2)n-COOH. Fatty acids are predominantly unbranched and those with even numbers of carbon atoms between 12 and 22 carbons long react with glycerol to form lipids (fat-soluble components of living cells) in plants, animals, and microorganisms. Fatty acids all have common names respectively lilk lauric (C12), MyrIstic (C14), palmitic (C16), stearic (C18), oleic (C18, unsaturated), and linoleic (C18, polyunsaturated) acids. The saturated fatty acids have no double bonds, while oleic acid is an unsaturated fatty acid has one double bond (also described as olefinic) and polyunsaturated fatty acids like linolenic acid contain two or more double bonds. Lauric acid (also called Dodecanoic acid) is the main acid in coconut oil (45 - 50 percent) and palm kernel oil (45 - 55 percent). Nutmeg butter is rich in myristic acid (also called Tetradecanoic acid ) which constitutes 60-75 percent of the fatty-acid content. Palmitic acid(also called Hexadecylic acid ) constitutes between 20 and 30 percent of most animal fats and is also an important constituent of most vegetable fats (35 - 45 percent of palm oil). Stearic acid ( also called Octadecanoic Acid) is nature's most common long-chain fatty acids, derived from animal and vegetable fats. It is widely used as a lubricant and as an additive in industrial preparations. It is used in the manufacture of metallic stearates, pharmaceuticals, soaps, cosmetics, and food packaging. It is also used as a softener, accelerator activator and dispersing agent in rubbers. Oleic acid (also called octadecenoic acid) is the most abundant of the unsaturated fatty acids in nature. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PRICES |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||