PRODUCT IDENTIFICATION
7758-05-6
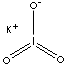
KIO3
214.00
H.S. CODE
2829.90
TOXICITY
CLASSIFICATION
GENERAL DESCRIPTION
Iodine is a nonmetallic halogen element in Group 17 of periodic table; atomic number 53; atomic mass 126.9; melting point ca 114 C; boiling point ca 184 C; specific gravity 4.93 g/cm3; oxidation states: 7,5,1,-1; [Kr] 4d10 5s2 5p5. Iodine is a nearly black poisonous, corrosive solid at room temperature and readily sublimes to a deep violet vapour, the colour of which is responsible for its name from Greek. It is insoluble in water, soluble in common solvents. Iodine is required in small amounts in human body for the function of the thyroid gland. Iodine forms many important compounds of iodine such as iodine(V)oxide, potassium iodide, iodine trichloride and iodoform of an Iodine containing organic compound.
The related name, iodate indicates any salt of iodic acid containing the IO3- radical; KIO3 (potassium iodate) and NaIO3 (sodium iodate) are the most important salts. Whereas, iodide indicates any compound of iodine with a more electropositive element or group such as such as CH3CH2I or any binary compound of iodine which may contain the I- radical and which may be considered to be derived from hydriodic acid (HI); KI and NaI are examples.
Dietary iodine is reduced to iodide, absorbed in the intestines, and later taken up from the bloodstream by the thyroid gland for incorporation into thyroid hormones. Iodine is applied in the treatment of thyrotoxic crisis to produce a thyroid gland of firm texture suitable for operation, it avoids the increased vascularity and friability of the gland with increased risk of haemorrhage. Iodine has powerful bactericidal activity. It is used is used for disinfecting skin and for the treatment of minor wounds and abrasions. Iodine has been used in the purification of drinking water in case of amoebicidal and bactericidal emergencies. Iodine is used as a component in germicides and disinfectants with surfactants to carry iodine. Iodine is used in the treatment of herpes simplex, keratoscleritis and preventing the development of goitre.
Inorganic iodide compounds are soluble in water and hygroscopic except a few inorganic iodides such as copper iodide. Their refractive indexes and specific gravities are higher than the corresponding chlorine and bromine analogues. The important iodides commercially are potassium iodide (KI), sodium iodide (NaI), hydrogen iodide (HI), and polyiodides.
Potassium and sodium Iodide are used in photography and as analytical reagents. They are used in the measurement of the energy of gamma rays, by measuring the amplitude of pulses of light generated by electrons which are excited by the gamma rays. They are used as nutrition supplements to prevent goitre and other iodine deficiency in human body. They are used in organic synthesis as well. Potassium iodide has been used as a mucolytic agent. Potassium iodide is used as a heat stabilizer and a catalyst in synthetic fiber manufacturing.
Hydriodic acid is the aqueous pale yellow solution of gas hydrogen iodide; the solution of 59% hydrogen iodide has constant-boiling. It is a strong acid and reducing agent used as raw materials for pharmaceuticals. analytical reagent as well as in organic synthesis and making iodine salts.
Inorganic iodate compounds, prepared generally by the oxidation of iodine with iodic acid or by electrolytic oxidation of iodide solutions, are stable oxidizers at room temperature though they lose oxygen at higher temperatures. Iodic acid (hydrogen iodate), a white crystalline powder, is a strong inorganic acid; highly corrosive oxidizing agent; decomposes at 110 C. It is used as a reducing agent in organic synthesis. Metallic iodates are explosive or flammable when contact with organic combustible materials. The important iodates commercially are potassium iodate and calcium iodate; white, odorless crystalline powder soluble in water, insoluble in alcohol. They are used as analytical reagents and in the manufacture of disinfectants, antiseptics, deodorants, medicines and other iodine compounds as well as oxidation of sulphur dyes. They are used in baking ingredient conditioner and as animal feed and food supplement for the treatment of their deficiency.
Potassium periodate is a white crystal or granular powder; soluble in hot water; slightly soluble in cool water and KOH solution; insoluble in alcohol; decompose at high temperature. It is used as a powerful oxidising agent in organic synthesis. It is used in colorimetric estimation of manganese.PHYSICAL AND CHEMICAL PROPERTIES
3.89
5 - 8 (5% Solution)
REFRACTIVE INDEX
APPLICATIONS
food industry, manufacturing of iodine compounds, animal feed supplement, oxidation of sulphur dyes, baking ingredient conditioner, photography, heat stabilization in nylon tire cord, medicine.
FCC IV (or 10% CALCIUM STEARATE ADDED)
APPEARANCE
White free-flowing crystals
KIO3
99.0 - 101.0%
ClO3
0.1% max
IODIDE
20ppm max
H2O
0.5% max
As
3ppm max
20ppm max
5 - 8 (20% Solution)
ACS
APPEARANCE
White free-flowing crystals or powder
ASSAY
99.0% min
INSOLUBLE MATTER
0.2% max
LOSS ON DRYING
0.2% max
pH
6 - 9 (5% solution)
CHLORIDE & BROMIDE
0.01% max
IODATE
3ppm max
N
0.001% max
PO4
0.001% max
SO4
0.005% max
Ba
0.002% max
Ca
0.002% max
Mg
0.001% max
Na
0.005% max
Fe
3ppm max
5ppm max