| L-VALINE, L-ISOLEUCINE | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
GENERAL DESCRIPTION | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Valine, Isoleucine and Leucine are all branched-chain amino acids which can be burned for fuel in the cells to promotes muscle recovery when in balance with each other most effective. Only the L-stereoisomers appear in mammalian protein. They play a role in wound healing and the growth of new tissue. These branched amino-acids are needed to increase the bio-availability of complex carbohydrate intake and are absorbed by the muscle cells for anabolic muscle building activity. Branched amino-acids (Valine, Isoleucine and Leucine ) with tryptophan and phenylalanine (aromatic side chain amino acids) contribute to the structure of protein by the tendency of its side chain composed only of carbon and hydrogen to participate in hydrophobic interactions which determines the tertiary structure of the peptide chain. Isoleucine, chemically 2-amino-3-methylvaleric acid, is a monocarboxylic amino acid occurring in most dietary proteins. It is necessary for optimal growth in infants and for nitrogen equilibrium in human adults. Leucine, chemically 2-amino-4-methylvaleric acid, is the isomer of isoleucine. Their methyl branch positions are difference, which show different properties of them. Norleucine, chemically 2-aminohexanoic acid, is the unbranched isomer. The prefix nor- describes normal structure which has no branched chain of carbon atoms. (In case of norepinephrine, it has one less methylene group than its homologue, epinephrine). Norleucine is a nonessential amino acid extracted from the leucine fraction of the decomposition of the proteins of nervous tissue. Commercial norleucine is synthesized for peptidomimetics to prepare unnatural and unusual amino acids and amino acid analogs as well as to modify peptides. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
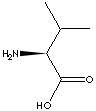
L-VALINE |
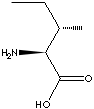
L-ISOLEUCINE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CAS
NO. : 72-18-4 |
CAS
NO. : 73-32-5 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| APPLICATIONS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Nutritional Supplements | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SPECIFICATION | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
L-Valine (FCC IV
GRADE)
L-Isoleucine(FCC IV GRADE)
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SYNONYMS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
L-Valine (S)-(+)-Valine; Val; V; Valine; L-(+)-valine; L-2-Amino-3-methylbutyric acid; 2-Aminoisovaleric acid; 2-Amino-3-methylbutyric acid; L-Isoleucine (2S,3S)-(+)-Isoleucine; Ile; I; Isoleucine; L-2-Amino-3-methylvaleric Acid; L-Isoleucine; (2S)-2-Amino-3-methylpentanoic acid; 2-Amino-3-methylvaleric acid; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
GENERAL DESCRIPTION OF AMINO ACID |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Amino Acid is any of the organic compounds in which one (or more ) amino group (-NH2) and one (or more ) carboxylic acid group (-COOH) are both present with general formula R-CH(NH2)COOH containing carbon, hydrogen, oxygen, nitrogen, and in certain cases sulfur atoms. Two groups attached to the same carbon (called the alpha-carbon atom at the end of the compound) are polymerized to form peptides and proteins. The amine group is protonated to form -NH3+ at low pH. The carboxylic acid group is deprotonated to form -CO2- at high pH. The carbon atom in the carboxyl group of one amino acid binds covalently to the nitrogen atom in the amino group of another amino acid to form a peptide bond with the release of a water molecule. Proteins are synthesized through the covalent chemical polypeptide bonds. The sequence of these amino acids in the protein polypeptides determines the shape, properties, and hence biological role of the protein that function as chemical messengers and as intermediates in metabolism. Proteins are composed of various proportions of about 20 commonly occurring amino acids. Plants or other biological systems can synthesize amino acids from simple inorganic compounds, but animals rely on adequate supplies in their diet. More than 100 common amino acids occur in plants or in other microorganic systems. The 20 amino acids commonly found in animals are Alanine, Arginine, Asparagine, Aspartic Acid, Cysteine, Glutamic Acid, Glutamine, Glycine, Histidine, Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Proline, Serine, Threonine, Tryptophan, Tyrosine, and Valine. Many of the amino acids can be synthesized in the human or animal body from other cellular metabolites when needed (called Non-essential Amino Acids). Animals are not able to synthesize some amino acids necessary in metabolism in sufficient quantities. It must therefore be present in the diet (called Essential Amino Acids). In man, these essential amino acids are Arginine, Histidine, Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Threonine, Tryptophan and Valine. Valine, Isoleucine and leucine are all branched-chain amino acids, which can be burned for fuel in the cells to promotes muscle recovery when in balance with each other most effective. Only the L-stereoisomers appear in mammalian protein. They play a role in wound healing and the growth of new tissue. These branched amino-acids are needed to increase the bio-availability of complex carbohydrate intake and are absorbed by the muscle cells for anabolic muscle building activity. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
GENERAL PROPERTIES OF AMINO ACIDS |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||