PRODUCT IDENTIFICATION
2235-46-3
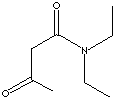
157.21
H.S. CODE
TOXICITY
N,N-Diethyl-3-oxobutyramide; N,N-Diethylacetessigsäureamid (German); Acetoacetic acid diethylamide; N,N-Diéthylacétoacétamide (French); N,N-Dietilacetoacetamide (Italian); Acetoacetdiethylamide; N,N-Diethylacetylacetamide; 1-Diethylcarbamoyl-2-propanone;
CLASSIFICATION
ACETOACETIC ACID DERIVATIVES / AMIDES /
PHYSICAL AND CHEMICAL PROPERTIES
clear to yellow liquid
213 - 218 C
0.993 - 0.997
REFRACTIVE INDEX
94 C
Stable under ordinary conditions
GENERAL DESCRIPTION & APPLICATIONS
Acetoacetone is a beta-diketone (1,3-diketone) which two ketones are separated only by one carbon. The beta-ketone is stable as a conjugated enol rather than alpha-diketone due to the delocalization which makes the counterion more stable and less likely to regain the proton. Ascorbic acid is an example of enol compound. Enol compounds form complexes with many transition metal ions. These compounds are readily soluble in organic solvents. They are widely used as chelating agents, ligands, and catalyst precursors.
Acetoacetic acid and its esters contain active methylene groups which have relatively acidic alpha-protons due to H atom adjacent to two carbonyl groups. The reactivity of its methylene group provide the sequence of reactions of alkylation, hydrolysis of the esters and decarboxylation resulting in substituted ketones. The methylene group can be reacted to form amino-carbonyl compounds. Acetoacetates are important aliphatic parts adjoining azo dyes and pigments. Acetoacetic acid is unstable and decompose to acetone and carbon dioxide at room temperature. Aacetoacetate is one of ketone bodies which are the end-products of rapid or excessive fatty acid breakdown in the human body.
Diethyl Acetoacetamide has the multi functionality including that of acetoacetate reactive hydrogen atom on the carbon alpha to both carbonyl groups and amide. It is used to forms a large class of target products including amino acids, drugs, colorants, lacquers, perfumes, pesticides, and unsaturated polyesters.
APPEARANCE
clear to yellow liquid
ASSAY
98.0% min
WATER
0.5% max
200kgs in drum