| TRICHLOROTRIFLUOROETHANE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 76-13-1 |
|
| EINECS NO. | 200-936-1 | |
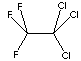
| FORMULA | CCl2FCClF2 | |
| MOL WT. | 187.38 | |
| H.S. CODE | ||
|
TOXICITY |
Oral rat LD50: 43 gm/Kg | |
| SYNONYMS | Freon 113; Fluorocarbon 113; Halocarbon 113; | |
| 1,1,2-Trichlorotrifluoroethane; Trichloro 1,2,2-trifluoroethane; 1,1,2-Trifluorotrichloroethane; 1,1,2-Trifluoro-1,2,2-trichloroethane; Freon TF Solvent (Ashland); Racon 113 (Racon Inc.); Freon TF/PCA (Dupont); Rho-tron PCA; | ||
|
RAW MATERIALS |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | Colorless, volatile liquid | |
| MELTING POINT | -36 C | |
| BOILING POINT | 48 C | |
| SPECIFIC GRAVITY | 1.56 | |
| SOLUBILITY IN WATER | Slightly soluble | |
|
SOLVENT SOLUBILITY |
Soluble: alcohol, ether, benzene | |
| pH |
|
|
| VAPOR DENSITY | 6.47 | |
| AUTOIGNITION |
|
|
| NFPA RATINGS | Health: 2 Flammability: 0 Reactivity: 0 | |
|
REFRACTIVE INDEX |
||
| FLASH POINT |
|
|
| STABILITY | Stable at normal conditions | |
|
APPLICATIONS |
||
| Used as a solvent and refrigerant; It is used in fire extinguishers and as a blowing agent. It has a wide range of cleaning applications and vapor degreasing. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
Colorless, volatile liquid | |
|
ASSAY |
99.9% max |
|
|
ACID NUMBER |
0.003 (KOH mg/gr) |
|
|
WATER |
10ppm max |
|
|
COLOR (APHA) |
10 max |
|
| TRANSPORTATION | ||
| PACKING | ||
| HAZARD CLASS | ||
| UN NO. | ||
| GENERAL DESCRIPTION OF CFCs | ||
|
The most common commercial CFCs (chlorofluorocarbons), aliphatic organic compounds composed of carbon, fluorine, chlorine, and hydrogen, are trichlorofluoromethane (commonly known as CFC-11 or F-11) and dichlorodifluoromethane (CFC-12 or F-12), chlorodifluoromethane (22), dichlorotetrafluoroethane (114), and trichlorotrifluoroethane (113). CFCs are also known by the trade name Freon. They are nontoxic, noncorrosive and nonflammable and can be readily converted from a liquid to a gas and vice versa. In addition to these properties, their low boiling points, low surface tension, and low viscosity make them to be used extensively as aerosol-spray propellants, refrigerants, solvents, cleansing agents for electrical and electronic components, and foaming agents in shipping-plastics manufacturing. The presence of fluorine atoms in CFCs molecules makes them extremely stable, inert compounds that are entirely harmless to humans. However, CFCs were identified CFCs as the major cause of ozone depletion in the upper atmosphere. Hydrochlorofluorocarbons (HCFCs), composed of carbon, hydrogen, chlorine and fluorine, are similar to CFCs but less destructive to ozone. They are used as replacements for CFCs. But they are also expected to be replaced by hydrofluorocarbons (HFCs) which do not contain chlorine, do not have any potential for the destruction of ozone. |
||
| GENERAL DESCRIPTION OF FLUORINE AND ITS COMPOUNDS | ||
|
Fluorine (Symbol : F; Atomic no. 9 ) is a yellowish, poisonous, corrosive gas under ordinary conditions. Fluorine becomes a yellow liquid upon cooling. It is the most reactive nonmetallic element and extremely powerful oxidizing agent. Because of its extreme reactivity, fluorine does not occur uncombined in nature. Fluorine occurs widely combined in the mineral fluorspar( fluorite, the chief commercial source), cryolite and apatite. The preparation of the free element is carried out by the electrolysis of a molten mixture of hydrogen fluoride, HF, and potassium fluoride, KF in the absence of water. Fluorine can be safely stored under pressure in cylinders of stainless steel if the valves of the cylinders are free from traces of organic matter. The outstanding oxidizing properties of the elemental gas are used in some rocket fuels. The element may be used for the fluorination of organic compounds with appropriate precautions. The element is used for manufacturing various fluorides including chlorine trifluoride ans cobalt(III) fluoride which are important fluorinating agents for organic compounds, sulfur(VI) fluoride used as a gaseous electrical insulator. Boron trifluoride and antimony trifluoride like hydrogen fluorides are important catalysts for alkylation reactions used to prepare organic compounds. Sodium fluoride (NaF) is used to treat dental caries and is often used for the fluoridation of drinking water to reduce tooth decay (However, there are reports of an accompanying risk of fluoride toxicity ). The element is also used for the preparation of uranium(VI) fluoride, utilized in the gaseous diffusion process of separating uranium-235 from uranium-238 (natural uranium) for reactor fuel. The importance of fluorine lies largely in its extreme ability to attract electrons and to the small size of its atoms, which can be attributed to form many stable complexes with positive ions like hexafluorosilicate(IV) and hexafluoroaluminate(III). Fluorine derivatives of hydrocarbons (compounds of carbon and hydrogen) are useful extensively as aerosol-spray propellants, refrigerants, solvents, cleansing agents for electrical and electronic components, and foaming agents in shipping-plastics manufacturing. Useful plastics with non-sticking qualities, such as polytetrafluoroethylene ( known by the trade name Teflon), are readily made from unsaturated fluorocarbons. A solution of hydrogen fluoride gas in water is called hydrofluoric acid, largely consumed for cleaning metals and for polishing, frosting, and etching glass. Hydrofluoric acid is also used as a catalyst for alkylation reactions. The chemical reactions are similar to those in the sulfuric acid process, but it is possible to avoid refrigeration. (In sulfuric acid alkylation, refrigeration is necessary because of the heat generated by the reaction). |
||