|
PARALDEHYDE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 123-63-7 |
|
| EINECS NO. | 204-639-8 | |
| FORMULA | C6H12O3 | |
| MOL WT. | 132.16 | |
| H.S. CODE | 2912.50 | |
|
TOXICITY |
Oral rat LD50: 2711 mg/kg | |
| SYNONYMS | 1,3,5-Trimethyl-2,4,6-trioxane; Paracetaldehyde; | |
| Paracetaldehyde; 2,4,6-Trimethyl-1,3,5-Trioxane; Trimethyl 1,3,5-Trioxane; PARAL; Acetaldehyde trimer; Acetaldehyde, Trimer; Elaldehyde; Paraacetaldehyde; 2,4,6-Trimethyl-s-trioxane; s-Trimethyltrioxymethylene; Paraldehyd (German); Paraldéhyde (French); Paraldeide (Italian); 2,4,6- Trimethyl-1,3,5-trioxaan (Dutch); 2,4,6- Trimetil-1,3,5-triossano (Italian); | ||
|
RAW MATERIALS |
|
|
|
CLASSIFICATION |
|
|
|
GENERAL DESCRIPTION |
||
|
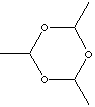
Paraldehyde (also called paracetaldehyde) , colourless highly flammable, disagreeable taste and pungent odored liquid, is a cyclic trimer of acetaldehyde contains a six-membered ring of alternating carbon and oxygen atoms with a hydrogen atom and a methyl group attached to each carbon atom. ( tetramer is metaldehyde, which has a similar structure to the trimer but with an eight-membered ring.). It boils at 124 C and melts at a temperature of 12.5 C. It is used as a used as a solvent for fats, oils, waxes, rubber and resins, as a substitute for acetaldehyde and as an intermediate for organic chemicals, dyestuffs, accelerators for vulcanizations, rubber oxidants, etc. It is a sedative-hypnotic drug But side-effects are expected like digestive upsets and, in large doses, prolonged unconsciousness. Paraldehyde is available on prescription only; paraldehyde should be used with caution in people with disease of the airways or lungs. Paraldehyde is commercially prepared the polymerization of acetaldehyde with a small amount of sulfuric acid; then neutralization with calcium carbonate and purifying by fractional distillation are followed. |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | clear to yellowish liquid with pleasant, sweet odor | |
| MELTING POINT | 12.5 C | |
| BOILING POINT | 124 C | |
| SPECIFIC GRAVITY | 0.995 | |
| SOLUBILITY IN WATER | Sparingly soluble | |
| pH |
| |
| VAPOR DENSITY | 4.5 | |
| AUTOIGNITION |
| |
| NFPA RATINGS | Health: 2 Flammability: 3 Reactivity: 1 | |
|
REFRACTIVE INDEX |
||
| FLASH POINT |
27.5 C | |
| STABILITY |
It is easily depolymerized to acetaldehyde | |
|
APPLICATIONS |
||
|
It is used as a used as a solvent for fats, oils, waxes, rubber and resins, as a substitute for acetaldehyde and as an intermediate for organic chemicals, dyestuffs, accelerators for vulcanizations, rubber oxidants, etc. It is a sedative-hypnotic drug |
||
| SALES SPECIFICATION | ||
| APPEARANCE |
clear to yellowish liquid | |
|
ASSAY (G.C) |
99.5% min | |
|
ACETIC ACID |
0.3% max | |
|
WATER |
0.1% max | |
| TRANSPORTATION | ||
| PACKING | 200kgs in drum | |
| HAZARD CLASS | 3.3 (Packing group : III) | |
| UN NO. | 1264 | |
| REMARKS (DESCRIPTION OF ALDEHYDE) | ||
|
Aldehydes are organic compounds containing -CHO radical, in which a carbon atom forms a solid bond with an oxygen atom and is also bonded to a hydrogen atom and another group denoted by R, which can be a second hydrogen atom, an alkyl group, or an aryl group. The most important and the simplest examples are methanal (formaldehyde), HCOH, and ethanal (acetaldehyde), CH3CHO.( In systematic chemical nomenclature, aldehyde names end with the suffix -al). Formaldehyde is used to make synthetic resins by reaction with phenols, urea, and melamine, as a chemical intermediate, as an embalming fluid, and as a disinfectant. Acetaldehyde is used chiefly to manufacture acetic acid. They are unpleasant-smelling liquids widely used in the chemical industry, whereas aromatic aldehydes frequently have pleasant smells and are used widely as flavourings and perfumes. An example is benzaldehyde (benzenecarbaldehyde, C6H5CHO), a derivative of benzene with an aldehyde group attached to the ring. It is a colourless oil smelling of almonds offer applications to both in perfumes and flavourings. Aldehydes are formed by oxidation of primary alcohols; further oxidation yields carboxylic acids. Aldehydes have certain characteristic addition and condensation reactions. Aldehydes can be reduced to primary alcohols (ketones to secondary alcohols). Aldehydes form cyanohydrins with hydrogen cyanide, acetals with alcohol, yellow-orange solid derivatives with DNP ( 2,4-dinitrophenylhydrazones) and undergo condensation reactions to yield oximes (compounds containing the group C:NOH), hydrazones (organic compounds containing the group =C:NNH2), and semicarbazones (organic compounds containing the unsaturated group =C:N.NH.CO.NH2). Aldehydes readily polymerize. |
||