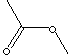
| METHYL ACETATE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PRODUCT IDENTIFICATION |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS NO. | 79-20-9 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EINECS NO. | 201-185-2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FORMULA | CH3COOCH3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MOL WT. |
74.08 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| H.S. CODE |
2915.39 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
TOXICITY |
Oral rat LD50: 5000 mg/kg | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SYNONYMS | Acetic acid, methyl ester; Methyl acetic ester; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Acetate de Methyle (French); Methyl Ester of Monoacetic Acid; Methyl Ethanoate; Methylacetaat (Dutch); Methylacetat (German); Methylester Kiseliny Octove (Czech); Metile (Acetato Di) (Italian); Octan Metylu (Polish); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
RAW MATERIALS |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CLASSIFICATION |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PHYSICAL STATE | clear liquid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MELTING POINT | -75 C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BOILING POINT |
57 - 58 C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SPECIFIC GRAVITY |
0.93 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SOLUBILITY IN WATER | 250 g/l | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| pH |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VAPOR DENSITY | 2.55 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AUTOIGNITION |
454 C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NFPA RATINGS | Health: 1; Flammability: 3; Reactivity: 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
REFRACTIVE INDEX |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FLASH POINT |
< 55 C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| STABILITY | Stable under normal conditions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
APPLICATIONS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Acetate is the
ester that an organic group replaces a hydrogen atom in -OH group of acetic acid
through reaction (typically condensation) with alcohols. Condensation is the
reaction in which two molecules having -OH groups are joined with eliminating a
water molecule from their -OH groups. They are produced by esterification reaction from acetic acid and the corresponding alcohol in the presence of strong acids like sulfuric acid. This reaction is reversible and acetate
can be hydrolyzed back into
alcohol and acetic acid in the presence of strong bases or strong acid, especially at elevated temperature.
The term acetate is also for the salt that
one or more of the hydrogen atoms of acetic acid are replaced by one or more
cations of the base, resulting in a compound containing the negative organic ion
of CH3COO-. Lower
acetate is a non-polar to weak polar aprotic solvent which have some solubility
portion in water. Its miscibility with water gets higher at elevated temperature.
Higher acetates have a
low
solubility in water and used as extraction solvents for fine chemicals
particularly for certain antibiotics. Organic acetates are
good solvents for a broad range of resins as they are miscible with almost all
common organic liquids. Due to their powerful solvency, high volatility and mild
odor, acetates are widely used as solvents for paints, coatings, adhesives, cellulose, plastics, fats,
wood stains. Additionally ether acetates series are also widely used as solvents. This surfactant-like structure provides the compatibility between water and a
number of organic solvents, and the ability to couple unlike phases. The main
products include ethyleneglycol
monoethyl ether acetate, ethyleneglycol monobutyl ether acetate,
and propyleneglycol monomethyl ether acetate.
Aromatic acetates
such as benzyl acetate are also useful solvent. Benzyl acetate has jasmine
like odor. Isoamyl acetate has a similar smell to both banana and pear. Acetates have characteristic fruity odor. They are used as component of perfumes
and flavorings.
They are used as chemical intermediate to manufacture pharmaceuticals, synthetic
flavorings, cleaners, and other organic compounds.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SALES SPECIFICATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| APPEARANCE |
Clear liquid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PURITY (GC) |
97.0% , 80.0% | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| WATER |
0.1% max | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
COLOR (Pt-Co) |
10 max | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TRANSPORTATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PACKING | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HAZARD CLASS | 3 (Packing Group: II) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UN NO. | 1231 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OTHER INFORMATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
European Hazard
Symbols: F, Risk Phrases: R 11-36-66-67, Safety Phrases: 16-23-29-33 FEMA No: 2676 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GENERAL DESCRIPTION OF SOLVENT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Solvent is a substance, usually a liquid, that acts as a dissolving agent or
that is capable of dissolving another substance. In solutions of solids or gases
in a liquid, the liquid is the solvent. In all other homogeneous mixtures (i.e.,
liquids, solids, or gases dissolved in liquids; solids in solids; and gases in
gases), solvent is the component of the greatest amount. The minor proportion
substances are called solutes. The solvent offers several functions during a
chemical reaction. It solves not only the substance that reacts with another one
to produce a new set of substances (reactant) but also the compound that
supplies the molecule, ion, or free radical, which is considered as the
attacking species in a chemical reaction (reagent). The solvent is conductive to
collisions between the reactants and reagents to transform the reactants to new
products. The solvent also takes roll of temperature control, either to provide
the energy of the colliding particles for speedy reaction and to absorb heat in
exothermic reaction. The appropriate solvent should be selected based on the
inactivity in the reaction conditions, dissolving the reagents as well as
reactants, appropriate boiling point and easy removal at the end of the
reaction.
Polarity The most common solvent is water. Other common solvents which dissolve substances that are insoluble (or nearly insoluble) in water are acetone, alcohol, formic acid, acetic acid, formamide. BTX, carbon disulfide, diemthyl sulfoxide, carbon tetrachloride, chloroform, ether, tetrahydrofuran, furfural, hexane and turpentine. They may be classified as polar and non-polar. Polar solvents, like water, have molecules whose electric charges are unequally distributed, leaving one end of each molecule more positive than the other. Usually polar solvent has O-H bond of which water (HOH), (CH3OH) and acetic acid (CH3COOH) are examples. Propanol, butanol, formic acid, formamide are polar solvents. Dipolar solvents which contain a C-O solid bond without O-H bond are acetone [(CH3)2C=O], ethyl acetate (CH3COOCH2CH3), methyl ethyl ketone, acetonitrile, N,N-dimethylformamide and diemthyl sulfoxide. Nonpolar solvents, like carbon tetrachloride (CCl4), benzene (C6H6), and diethyl ether ( CH3CH2OCH2CH3), have molecules whose electric charges are equally distributed and are not miscible with water. Hexane, tetrahydrofuran and methylene chloride are non-polar solvents. Polar solvents are hydrophilic but non-polar solvents are lipophilic. Polar reactants will dissolve in polar solvents. Non-polar solvents dissolve non-polar compounds best. Oil and water don't mix but separate into two layers. There are three measures of the polarity as "dipole moment", "dielectric constant" and "miscibility with water". Though low dipole moments and small dielectric constants indicates non-polar solvents, sharp boundaries between polar and non-polar solvents are not available. The polarity reflects the balance between a polar component (OH) and a non-polar hydrocarbon component, existing in the same molecule. If hydrocarbon character increases relatively, the polarity decreases. On an operational basis, solvents that are miscible with water are polar. Polar Protic and Dipolar Aprotic Protic refers to a hydrogen atom attached to an electronegative atom. Protic solvents can donate an H+ (proton) since they contain dissociable H+, such as hydrogen attached to oxygen as in a hydroxyl group, nitrogen as in a amine group. Examples are water, methanol, ethanol, formic acid, hydrogen fluoride and ammonia. Aprotic solvents don't has O-H bond but a C=O bond typically. Examples are acetone [(CH3)2C=O] and ethyl acetate (CH3COOCH2CH3). Polar protic solvents are useful in SN1 reaction, while polar aprotic solvents are SN2 reaction.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||