| DI-BASIC METHYL ESTERS BLEND OF DICARBOXYLIC ACID (DBME) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
GENERAL DESCRIPTION |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blends of Di-Basic Eesters derived from dicarboxylic acids are biodegradeable (environment friendly) solvents. They can replace volatile organic solvents in the application of paint stripper, inks, coatings, agriculture and adhesives. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PRODUCT IDENTIFICATION |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
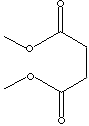
DIMETHYL SUCCINATE |
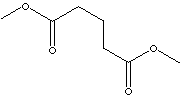
DIMETHYL GLUTARATE |
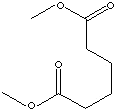
DIMETHYL ADIPATE |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CAS
RN: 106-65-0 |
CAS
RN: 1119-40-0 |
CAS
RN: 627-93-0 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
SALES SPECIFICATION |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GENERAL DESCRIPTION OF DICARBOXYLIC ACID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Dicarboxylic
acid is a compound containing two carboxylic acid, -COOH,
groups. Straight chain examples are shown in table. The
general formula is HOOC(CH2)nCOOH,
where oxalic acid's n is 0, n=1 for malonic acid, n=2 for succinic acid, n=3
for glutaric acid, and etc. In substitutive
nomenclature, their names are formed by adding -dioic'
as a suffix to the name of the parent compound. They
can yield two kinds of salts, as they contain two carboxyl
groups in its molecules. The range of carbon chain lengths is from 2, but the
longer than C 24 is very rare. The term long chain
refers to C 12 up to C 24 commonly. Carboxylic
acids have industrial application directly or indirectly
through acid halides, esters, salts, and anhydride forms,
polymerization, and etc. Dicarboxylic acids
can yield two kinds of salts
or esters, as they
contain two carboxyl groups in one molecule.
It is useful in a variety of industrial applications
include;
There are almost infinite esters obtained from carboxylic acids. Esters are formed by removal of water from an acid and an alcohol. Carboxylic acid esters are used as in a variety of direct and indirect applications. Lower chain esters are used as flavouring base materials, plasticizers, solvent carriers and coupling agents. Higher chain compounds are used as components in metalworking fluids, surfactants, lubricants, detergents, oiling agents, emulsifiers, wetting agents textile treatments and emollients, They are also used as intermediates for the manufacture of a variety of target compounds. The almost infinite esters provide a wide range of viscosity, specific gravity, vapor pressure, boiling point, and other physical and chemical properties for the proper application selections.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||