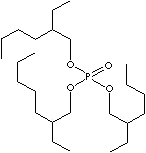
| TRIS(2-ETHYLHEXYL)PHOSPHATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 78-42-2 |
|
| EINECS NO. | 201-116-6 | |
| FORMULA | (C8H17O)3P(O) | |
| MOL WT. | 434.64 | |
| H.S. CODE | ||
|
TOXICITY |
||
| SYNONYMS | Trioctyl phosphate; Triethylhexyl phosphate; | |
| Phosphoric acid, tris(2-ethylhexyl) ester; TOF; 2-Ethylhexanol, phosphate Triester; 2-Ethyl-1-hexanol phosphate; Tris(2-ethylhexyl)phosphate; Tris(2-ethylhexyl)phosphat (German); Fosfato de tris(2-etilhexilo) (Spanish); Phosphate de tris(2-éthylhexyle) (French); | ||
| SMILES |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | clear to yellowish liquid | |
| MELTING POINT | -70 C | |
| BOILING POINT | 230 C (Decomposes) | |
| SPECIFIC GRAVITY | 0.920 - 0.93 | |
| SOLUBILITY IN WATER |
Insoluble |
|
|
SOLVENT SOLUBILITY |
|
|
| pH |
|
|
| VAPOR DENSITY | ||
| HENRY'S LAW |
|
|
| NFPA RATINGS | Health: 2 Flammability: 1 Reactivity: 0 | |
|
REFRACTIVE INDEX |
1.444 | |
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions | |
|
APPLICATIONS |
||
| Phosphoric acid alkyl esters are used as alkylation agent for nitrogen heterocyclic compounds and as catalysts to produce phenolic and urea resins. They are used flame retarding plasticizers for cellulose esters, lacquers, plastic and vinyl resins as well as dispersing agents in plastisols. They are used as solvents in liquid-liquid extractant or separation agent of metals. They are is used as heat exchange media, pigment grinding assistants and antifoaming agents. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
clear to yellowish liquid | |
|
ESTER CONTENT |
99.0% min |
|
|
ACIDITY (H3PO4) |
0.05 max |
|
| COLOR, HAZEN | 30 max | |
| WATER | 0.5% max | |
| TRANSPORTATION | ||
| PACKING |
190kgs in drum |
|
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: C, Risk Phrases: 34, Safety Phrases: 38 | ||
|
GENERAL DESCRIPTION OF PHOSPHORIC ACID |
||
| Phosphoric Acid (also called Orthophosphoric Acid) is a three atoms compound made up of phosphorus, oxygen, and hydrogen. It is prepared commercially by heating calcium phosphate rock with sulfuric acid; purer grades may be prepared by treating red phosphorus with nitric acid. Pure phosphoric acid is a crystalline solid melting point 42 C; in less concentrated form it is a colourless syrupy liquid. It is a tribasic acid and forms orthophosphate salts with either one, two, or all three of the hydrogens replaced by some other positive ion. It is used to make phosphates for fertilizers, electro chemical polishing and shaping, electroplating, metal cleaning and pickling in metal treatment. It is also used in the manufacture of industrial cleaning and derusting solutions, for production of other inorganic and organic phosphoric chemicals, for foundry resins, for production of paints, enamels and refractory, for textile processing, for water treatment, for antifreeze production. | ||