| GLYCOLIC ACID, n-BUTYLESTER | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 7397-62-8 |
|
| EINECS NO. | 230-991-7 | |
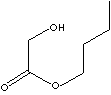
| FORMULA | HOCH2COOC4H9 | |
| MOL WT. | 132.16 | |
| H.S. CODE | ||
|
TOXICITY |
||
| SYNONYMS | Hydroxyacetic Acid butyl ester; Butyl hydroxyacetate; | |
| Glycolic acid n-butylester; Butylglykolat (German); Glicolato de butilo (Spanish); Glycolate de butyle (French); Butylglycolate; Polysolvan O; | ||
|
SMILES |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | clear liquid | |
| MELTING POINT | -26 C | |
| BOILING POINT |
186 - 190 C | |
| SPECIFIC GRAVITY | 1.015 - 1.025 | |
| SOLUBILITY IN WATER | ||
| pH |
| |
| VAPOR DENSITY | ||
|
HENRY'S LAW |
| |
|
NFPA RATINGS |
Health: 1; Flammability: 1; Reactivity: 0 | |
|
REFRACTIVE INDEX |
1.427 | |
| FLASH POINT |
75 C | |
| STABILITY |
Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
| Glycolic acid is the smallest alpha-hydroxy acid (AHA) which has a hydroxyl group on the carbon atom next to the acid group. If the hydroxy group is on the second carbon next to the acid group, it is called beta-hydroxy acid. Glycolic acid is a low mole weight compound but has dual functionality of alcohol and acid. Because of its small molecular weight and size, it has a better capability to penetrate skin. AHA is used extensively in cosmetics. It is known that it diminishes the lines on the skin and make skins look young by acting as a humectant to absorb moisture in air and by exfoliating action to break the bonds between dead skin cells, Pure glycolic acid is a white crystals; decomposes about 78 C; very soluble in water, alcohols, acetone, acetates; slightly soluble in ethyl ether; sparingly soluble in hydrocarbon solvents; polymerization occurs above 50 C. Commercially, it is available in crystal form as well as in aqueous solutions of various concentrations, usually 70 percent or less. Activating both the hydroxyl and carboxylic acid group, glycolic acid has the property to form metal complexes and can be used in cleaning applications and hard water dissolution. Aqueous solution grade is mainly used fro this cleaning application. Glycolic acid is an useful intermediate in organic synthesis including oxidation/reduction, esterification and formation of oligomers as well as long chain polymerization. Glycolic acid is naturally found in sugar beets, cane sugar, and unripe grapes. It is known that it diminishes the lines on the skin and make skins look young. Synthetic glycolic acid and its derivatives are used commercially in leather tanning and textile dyeing; as a flavouring agent and preservative in food processing, water treatment industries; pH control; and chemical intermediates as well as in household, industrial, electronics cleaning applications and cosmetics. They are also used in emulsion polymers, solvents and ink and paint additive to improve flow properties and impart gloss. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
clear liquid | |
| ESTER CONTENT |
97.0% min | |
| COLOR, APHA |
10 max | |
| WATER |
0.1% max | |
|
FREE ACID |
0.1% max | |
| TRANSPORTATION | ||
| PACKING | 200kgs in Drum | |
| HAZARD CLASS | ||
| UN NO. | 1993 | |
| OTHER INFORMATION | ||
| Hazard Symbols: , Risk Phrases: , Safety Phrases: 23-24/25 | ||