| GLUCONIC ACID | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 526-95-4 |
|
| EINECS NO. | 208-401-4 | |
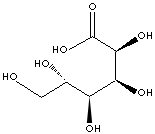
| FORMULA | CH2(OH)(CHOH)4COOH | |
| MOL WT. | 196.16 | |
|
H.S. CODE |
2918.16.1000 | |
| TOXICITY | Oral rat LD50: > 90 ml/kg | |
| SYNONYMS | D-Gluconic acid; Dextronic acid; Glosanto; | |
| 2,3,4,5,6-Pentahydroxyhexanoic acid; Gluconic acid; Glycogenic acid; Maltonic acid; Pentahydroxycaproic acid; | ||
| SMILES | O[C@@H]([C@@H]([C@@H](CO)O)O)[C@H](C(O)=O)O |
|
|
CLASSIFICATION |
Gluconate; Monosaccharide, Food additive, Chelating agent, Sugar acid, Souring agent, Sequestering agent |
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
Commercially available as a aq. solution |
|
| MELTING POINT | 131 C | |
| BOILING POINT | > 100 C (50% aq. solution) | |
| SPECIFIC GRAVITY | 1.23 - 1.25 (50% aq. solution) | |
| SOLUBILITY IN WATER | soluble | |
| SOLVENT SOLUBILITY | Slightly soluble in alcohol. Insoluble in ether and most organic solvents. | |
| pH |
1.8 (50% aq. solution) |
|
| log Pow | -1.87 (Octanol-water) | |
| VAPOR PRESSURE | 3.72E-10 (mmHg) | |
| HENRY'S LAW | 4.74E-13 (atm-m3/mole at 25 C) | |
| OH RATE | 4.23E-11 (cm3/molecule-sec at 25 C Atmospheric) | |
| VAPOR DENSITY | ||
|
AUTOIGNITION |
|
|
|
NFPA RATINGS |
Health: 1; Flammability: 0; Reactivity: 0 | |
|
REFRACTIVE INDEX |
||
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions. This material is incompatible with strong oxidizing agents, copper, Copper alloys. | |
|
GENERAL DESCRIPTION & EXTERNAL LINKS |
||
|
Gluconic acid is a polyhydroxycarboxylic acid
with six carbon length. It is derived from glucose by
oxidation of the aldehyde group on the C-1 to a carboxyl group. It is abundant
in plants, fruits and other foodstuffs. Commercially the physiological d-form
gluconic acid is prepared by fermentation process. It has a carboxylic group and
five hydroxy groups, and thus is a good chelator particularly in alkaline
conditions. Chelation is a chemical combination with a metal in complexes in
which the metal is part of a ring. Organic ligand is called chelator or
chelating agent, the chelate is a metal complex. The larger number of ring
closures to a metal atom is the more stable the compound. Chelation is applied
in metal complex chemistry, organic and inorganic chemistry, biochemistry, and
environment protection. It is used in chemotherapeutic treatments for metal
poisoning. Chelating agents offers a wide range of sequestrants to control metal
ions in aqueous systems. By forming stable water soluble complexes with
multivalent metal ions, chelating agents prevent undesired interaction by
blocking normal reactivity of metal ions. Heavy metals are chelated in alkaline
solution and their interferences are eliminated gluconic acid. Concentrated
gluconic acid solution contains certain lactone structure, a neutral cyclic
ester, showing antiseptic property. Gluconic acid and its derivatives (salts or
esters) are used in the formulation of pharmaceuticals, foods, and cosmetics as
mineral supplements to prevent the deficiency and as buffer salts. They are used
as ingredients in various hygienic products. In industrial applications, they
are used for scale removal in metal cleanings, industrial and household cleaning
compounds including mouth washer, metal finishing, water treatments, and as
paper and textile auxiliaries.
CAS RN of Salts: 299-27-4; 35087-77-5 (potassium salt), 527-07-1; 14906-97-9 (hydrochloride salt), 3632-91-5 (magnesium salt), 6485-39-8 (manganese salt), 10101-21-0 (strontium salt), 10361-31-6 (ammonium salt), 13005-35-1 (copper salt), 22830-45-1 (iron(+2) salt), 35984-19-1 (tin(+2) salt), 60007-93-4 (aluminum salt), 60816-70-8 (lithium salt), 82139-35-3 (zinc salt) Wikipedia Linking: http://en.wikipedia.org/wiki/Gluconic_acid http://www.inchem.org/documents/sids/sids/gluconates.pdf http://www.gmo-compass.org/ |
||
| SALES SPECIFICATION (50% AQ. SOLUTION) | ||
|
APPEARANCE |
clear to brown liquid | |
|
CONTENT |
50.0% min |
|
| MELTING POINT | apprx 12 C | |
| SPECIFIC GRAVITY | 1.23 - 1.26 | |
| REDUCING SUBSTANCES |
8.0% max |
|
|
TRANSPORTATION |
||
| PACKING | ||
| HAZARD CLASS | Not regulated | |
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: XI, Risk Phrases: 36/37/38. Safety Phrases: 26-28A-37-37/39-45 | ||