| CAS
NO. |
102-54-5 |
|
| EINECS
NO. |
203-039-3 |
| FORMULA |
(CH2)5Fe(CH2)5 |
| MOL
WT. |
186.04 |
| H.S.
CODE |
|
|
TOXICITY
|
Oral rat LD50:
1320 mg/kg |
| SYNONYMS |
Bis(cyclopentadienyl) iron;
Biscyclopentadienyliron; |
|
Dicyclopentadienyl iron;
Ferrotsen; Iron bis(cyclopentadiene); Iron
dicyclopentadienyl; Di-2,4-cyclopentadien-1-yliron; Ferrocene; Ferrotsen; Catane;
Other RN.: 51364-12-6, 55404-68-7, 856324-92-0, 856780-21-7 |
|
SMILES
|
C1=C[CH-]C=C1.C=1C=C[CH-]C1.[Fe+2] |
|
CLASSIFICATION
|
Catalysis, Metallocene
|
|
EXTRA
NOTES
|
Antiknock additive for gasoline |
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
yellow to orange
powder |
| MELTING POINT |
173
- 174 C |
| BOILING
POINT |
249
C |
| SPECIFIC GRAVITY |
|
| SOLUBILITY
IN WATER |
Insoluble |
|
SOLVENT
SOLUBILITY
|
Soluble in alcohol, ether and benzene |
| VAPOR DENSITY |
|
| log Pow |
3.28
(Octanol-water) |
| OH RATE
CONSTANT |
3.81E-10
(cm3/molecule-sec
at 25 C Atmospheric) |
| AUTOIGNITION
|
|
| NFPA
RATINGS |
|
| REFRACTIVE
INDEX
|
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions |
|
EXTERNAL LINKS
& GENERAL
DESCRIPTION
|
|
Wikipedia
Linking
Material
Safety Data Sheet
Google
Scholar Search
http://www.periodicvideos.com/
The
molecular video (University of Nottingham)
http://ideas.repec.org/
This paper reports catalytic effects of ferrocene on bonding, optical and
structural properties of diamond-like carbon (DLC) thin films grown on silicon
and quartz substrates by microwave surface-wave plasma chemical vapor
deposition. For film deposition, helium and methane gases were used as plasma
source. Bonding, optical and structural properties of the DLC films were
measured both with and without using ferrocene as a catalyst. The ferrocene
content in the DLC was confirmed by X-ray spectroscopy (XPS) measurement. The
optical band gap decreased from 2.7 eV (without ferrocene) to 1.6 eV (with
ferrocene). Raman spectra of the ferrocene content film shows that the G-peak
was more pronounced compared to the film without ferrocene. Results suggest that
appropriate concentration of ferrocene in DLC film helps to reduce the optical
band gap because of ferrocene-induced graphitization.
GENERAL
DESCRIPTION:
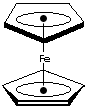
Ferrocene, yellow to orange crystal melting at 173 C, is an organometallic
compound of sandwich structure. It has two five membered carbon rings which
are
parallel, with the iron ion sandwiched between them. Its systematic name is
Di-[��]-Cyclopentadienyl Iron(II) or Bis(��5-Cyclopentadienyl) Iron. The bonding is between pi orbitals on the
rings and d-orbitals on the Fe2+ ion. It is used as a combustion control
additive in fuels, antiknock agent in gasoline,
and for heat stabilization in greases and plastics. It is used as a catalyst for the synthesis of ammonia. Ferrocene derivatives, can be described as a multi-electron transfer, are useful
for following fields:
- Stating
material for more coordination of organometallic-complexes
- Gasoline
additive to prevent "knocking" in motors
- Redox
mediators in biosensor
- Catalysts for the oxidative organic synthesis
- Molecular chemistry
- Disease diagnosis and treatment
- Water
treatment
- Photolysis chemistry
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
yellow to orange
powder |
|
CONTENT
|
99.0%
min
|
|
FREE-Fe
|
100ppm
max
|
|
INSOLUBLES
|
0.1%
max (in benzene)
|
|
WATER
|
0.1%
max
|
| TRANSPORTATION |
| PACKING |
25kgs
in drum |
| HAZARD CLASS |
4.1 (Packing
Group: II) |
| UN
NO. |
1325 |
| SAFETY
INFORMATION |
|
Hazard
Symbols: F XN, Risk Phrases: 11-22, Safety Phrases:
16 |
|
GENERAL
DESCRIPTION OF METALLOCENE
|
|
Metallocene is a compound consisting of parallel ring system ligand bound to a
metal. Ligand, in organometallic chemistry, is a molecule that donates or
accepts a pair of electrons to form a coordinate covalent bond with the central
metal atom of a coordination complexes and organometallic compounds; can be
further distinguished by the orbitals used in bond formation. There can be
numerous organometallic coordination compounds distinguished by number of
cyclopentadienyl rings (with or without substituents) bonded to a central
transition-metal atoms and the types of transition-metals and the type of the
bridge. Metals known to form metallocene complexes are titanium, zirconium,
hafnium, vanadium, chromium, molybdenum, tungsten, manganese, iron, ruthenium,
osmium, cobalt, rhodium, and nickel. Metallocenes possess the transition-metal
atoms whose bonding involves overlap of ns, (n - 1)d, and np orbitals of the
metal with molecular orbitals of appropriate symmetry of each aromatic
(bis-cyclopentadienyl) rings. The commonest metallocene is ferrocene, yellow to
orange crystal melting at 173 C, is an organometallic compound of sandwich
structure. It has two five membered carbon rings which are parallel, with the
iron ion sandwiched between them. The equivalent bonding of all 5 carbon atoms
of each cyclopentadienyl ring in ferrocene is denoted as eta-5. Its systematic
name is Di-[��]-Cyclopentadienyl Iron(II) or Bis(��5-Cyclopentadienyl) Iron. The
bonding is between pi orbitals on the rings and d-orbitals on the Fe2+ ion. It
is used as a combustion control additive in fuels, antiknock agent in gasoline,
and for heat stabilization in greases and plastics. It is used as a catalyst for
the synthesis of ammonia. Metallocenes having catalytic site in systematic
molecular structure are used as polymerization catalyst to produce uniform
polymer with unique structures and physical properties. Intensive study of
metal-carbon bonds have been developed in the field of electrochemical
techniques, high-temperature chemistry, photolysis chemistry, structural
chemistry, organic light-emitting devices, biochemistry and pharmaceutical manufacturing. |
|
PRICE
INFORMATION |
|
|
