PRODUCT IDENTIFICATION
TOXICITY
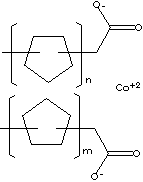
SMILES
CLASSIFICATION
EXTRA NOTES
UN2001 [Flammable solid]
PHYSICAL AND CHEMICAL PROPERTIES
> 300 C
0.921
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
PubChem Compound Summary - COBALT NAPHTHENATE
KEMI
- Swedish Chemicals Agency
Naphthenates are sorts of carboxylic acids which contain saturated cyclic
hydrocarbons such as cyclopentane and cyclohexane; acids of this kind are known
as naphthenic acids. The acids are separated from the gas oil fraction in
petroleum distilling. No such pure production occurs in Sweden, nor are the
salts produced here. About 5,000 tonnes of naphthenates were produced in the USA
in 1988. The most widely used naphthenates are certain metallic soaps. These
soaps are soluble, not in water but in many organic substances. The metallic
naphthenates are yellowish-brown and, depending on metallic content, viscous
liquids, sticky compounds or soft, solid substances. The metallic content can
vary between about 6% and about 40%. Naphthenates are mostly used as an ingredient in paints, to accelerate the
drying of the painted surface. When the binder in paint comes into contact with
atmospheric oxygen, the intention is for the binder to cross-link and become a
solid layer, i.e. to set. The metallic part of the siccative accelerates
absorption of atmospheric oxygen, catalyses the formation of free radicals, and
the binder reacts – dries – more rapidly. Most often a combination of two
metallic naphthenates is used to achieve rapid drying of the surface and
after-setting in depth. Calcium/lead naphthenate mixtures have been common, but
since the 1970s lead has been increasingly superseded by zinc and zirconium.
Lead, however, gives better after-setting at temperatures below 10ºC. Thus it is
the metallic part which decides how the naphthenate functions. The naphthenate
acid contributes by dissolving the substance in oils and other non-polar
substances. Metallic soaps applied to oils have a gelling effect – the lubricant
oil turns into lubricant grease. Naphthenates are also used in this way.
Local:
Naphthenic acid is a complex of carboxylic acids(
various low-molecular-weight fatty acids, believed to have cyclopentane ring
mainly.) obtained as a by-product of
petroleum refining; with a variable composition and ingredients; generally 180
- 350 mole wt. It is used in deicing, dust control, wood preservative and road
stabilization. Naphthenic acid and metallic
naphthenates have industrial applications in synthetic detergents, solvent
additives for paint, varnish,
oils and resins. They are used as lubricants, corrosion inhibitors and fuel
additives. They are used as wood preservatives; catalysts; insecticides;
wetting agents; lubricating oil additive.
Paint driers are substances put into paint to make dry quickly. They are metallic salts of low-molecular-weight (chiefly C8) fatty acids or naphthenic acids. Naphthenic acid is a complex of carboxylic acids( various low-molecular-weight fatty acids believed to have cyclopentane ring mainly). Hydrocarbon parts take oxygen in air and metals act as catalyst to speed up the oxidative coating. Cobalt is the most useful. It is a powerful oxidation catalyst and can keep whiteness. Auxiliary metals should be added to prevent surface wrinkling after drying. Primary metals which can replace for cobalt are zirconium, lead, cerium and iron and auxiliary metals are like calcium, manganese, barium, zinc, lithium.
APPEARANCE
3.8 - 4.2% or 9.8 - 10.2%
HAZARD OVERVIEW
Combustible Liquid, Carcinogen, Target Organ Effect, Skin and respiratory sensitizer. Target Organs: Thyroid., Heart, Male reproductive system., Blood
GHS
Danger
PICTOGRAMS


HAZARD STATEMENTS
H226
Flammable liquid and vapour.
H303 May be harmful if swallowed.
H304
May be fatal if swallowed and enters airways.
H317 May cause
an allergic skin reaction.
H320 Causes eye irritation.
H334
May cause allergy or asthma symptoms or breathing difficulties if
inhaled.
PRECAUTIONARY STATEMENTS
P261
Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray.
P280 Wear
protective gloves.
P301 + P310 IF SWALLOWED: Immediately call
a POISON CENTER or doctor/ physician.
P305 + P351 + P338 IF IN
EYES: Rinse cautiously with water for several minutes. Remove contact
lenses, if present and easy to do. Continue rinsing.
P331 Do
NOT induce vomiting.
P342 + P311 If experiencing respiratory
symptoms: Call a POISON CENTER or doctor/ physician.
![]() T
Toxic
T
Toxic![]() N
Dangerous for the environment
N
Dangerous for the environment
RISK PHRASES
36/38 Irritating to eyes and skin
42/43 May cause sensitization by inhalation and skin contact
49 May cause cancer by inhalation
50/53 Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment
SAFETY PHRASES
45 In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
53 Avoid exposure - obtain special instruction before use
60 This material and/or its container must be disposed of as hazardous waste
61 Avoid release to the environment. Refer to special instructions safety data sheet
PRICE INFORMATION