| CHLORACETYL CHLORIDE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 79-04-9 |
|
| EINECS NO. | 201-171-6 | |
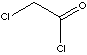
| FORMULA | ClCH2COCl | |
| MOL WT. | 112.94 | |
| H.S. CODE | 2915.90 | |
|
TOXICITY |
Oral rat LD50: 208 mg/kg | |
| SYNONYMS | Chloroacetyl chloride; Monochloroacetyl chloride; | |
| Chlorid kyseliny chloroctove; Chlorure de Chloracetyle (French); | ||
|
SMILES |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
Clear to slightly yellow fuming liquid | |
| MELTING POINT | -23 C | |
| BOILING POINT |
105 C | |
| SPECIFIC GRAVITY | 1.415 - 1.42 | |
| SOLUBILITY IN WATER | Rapidly hydrolysis | |
| pH |
| |
| VAPOR DENSITY | ||
|
AUTOIGNITION |
| |
|
REFRACTIVE INDEX |
| |
|
NFPA RATINGS |
Health: 3 Flammability: 0 Reactivity: 1 | |
| FLASH POINT | > 100 C | |
| STABILITY | Stable under ordinary conditions. but will fume when exposed to atmospheric moisture to form HCl and acetic acid. | |
|
DESCRIPTION AND APPLICATIONS |
||
Chloracetyl
Chloride is a clear
to slightly yellowish liquid with a strong pungent
odor similar to that of hydrochloric acid; melting point
23 C; boiling point 105 C. Chloracetyl
chloride, a
bifunctional chloride, offers following molecules for
the end products of agrochemicals,
pharmaceuticals, dyes, textile auxiliaries, paper
modifiers, plastic additives, and peroxide
compounds.
|
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
Clear liquid | |
|
ASSAY |
99.0% min | |
| ACETYL CHLORIDE |
0.5% max | |
|
COLOR |
30 max, Hazen | |
| TRANSPORTATION | ||
| PACKING | 275kgs in drum | |
| HAZARD CLASS | 3 (Packing group: I) | |
| UN NO. | 1752 | |
| OTHER INFORMATION | ||
| Hazard Symbols: T C N, Risk Phrases: 14-23/24/25-35-48/23-50, Safety Phrases: 7/8-9-26-36/37/39-45-61 | ||
|
GENERAL DESCRIPTION OF ACYL HALIDE |
||
| Acyl is a radical formed from an organic acid by removal of a hydroxyl group. The general formula of acyl compound is RCO-. Acyl halide is one of a large group of organic substances containing the halocarbonyl group, have the general formula RCO·X, where X is a halogen atom (fluorine, chlorine, bromine, iodine, and astatine) and R may be aliphatic, alicyclic, aromatic, and H etc. In substitutive chemical nomenclature, their names are formed by adding '-oyl' as a suffix to the name of the parent compound; ethanoyl chloride, CH3COCl, is an example. The terms acyl and aroyl halides refer to aliphatic or aromatic derivatives, respectively. Acyl halides are made by replacing the -OH group in carboxylic acids by halogen using halogenating agents. They react readily with water, alcohols, and amines and are widely used in organic synthetic process whereby the acyl group is incorporated into the target molecules by substitution of addition-elimination sequence called acylation reaction. Acylation reaction involves substitution by an electron donor (nucleophile) at the electrophilic carbonyl group (C=O). Common nucleophiles in the acylation reaction are aliphatic and aromatic alcohols, both of which give rise to esters and amines (RNH2) which give amides. The carboxylic acid (X = OH) itself can function as an acylating agent when it is protonated by a strong acid catalyst as in the direct esterification of an alcohol. Two common acylation agents, with the general formula RCOX, are acid halides (X = halogen atom) and anhydrides (X = OCOR). Schotten-Baumann reaction is an acylation reaction that uses an acid chloride in the presence of dilute alkali to acylate the hydroxyl and amino group of organic compounds. There are also other acylating agents. Chloride is used in agrochemicals and pharmaceuticals manufacturing. Acyl halide compounds are used as an intermediate for agrochemicals and pharmaceuticals, dyes, and peroxide compounds as well as textile auxiliaries, emulsifiers. | ||