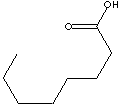
| CAPRYLIC ACID |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PRODUCT IDENTIFICATION |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS NO. | 124-07-2 |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EINECS NO. | 204-677-5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FORMULA | CH3(CH2)6COOH | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MOL WT. | 144.21 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| H.S. CODE | 2915.50 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
TOXICITY |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SYNONYMS | C-8 Acid; Neo-fat 8; n-Caprylic Acid; Capryloate; Octoic acid; Octic acid; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1-Heptanecarboxylic acid; n-Octanoic Acid; n-Octic acid; n-Octylic acid; Octanoic Acid; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CLASSIFICATION |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GENERAL DESCRIPTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Carboxylic acid is an organic compound whose molecules contain carboxyl group
and have the condensed chemical formula R-C(=O)-OH in which a carbon atom is
bonded to an oxygen atom by a solid bond and to a hydroxyl group by a single
bond), where R is a hydrogen atom, an alkyl group, or an aryl group. Carboxylic
acids can be synthesized if aldehyde is oxidized. Aldehyde can be obtained by
oxidation of primary alcohol. Accordingly, carboxylic acid can be obtained by
complete oxidation of primary alcohol. A variety of Carboxylic acids are
abundant in nature and many carboxylic acids have their own trivial names.
Examples are shown in table. In substitutive nomenclature, their names are
formed by adding -oic acid' as the suffix to the name of the parent compound.
The first character of carboxylic acid is acidity due to dissociation into H+
cations and RCOO- anions in aqueous solution. The two oxygen atoms are
electronegatively charged and the hydrogen of a carboxyl group can be easily
removed. The presence of electronegative groups next to the carboxylic group
increases the acidity. For example, trichloroacetic acid is a stronger acid than
acetic acid. Carboxylic acid is useful as a parent material to prepare many
chemical derivatives due to the weak acidity of the hydroxyl hydrogen or due to
the difference in electronegativity between carbon and oxygen. The easy
dissociation of the hydroxyl oxygen-hydrogen provide reactions to form an ester
with an alcohol and to form a water-soluble salt with an alkali. Almost infinite
esters are formed through condensation reaction called esterification between
carboxylic acid and alcohol, which produces water. The second
reaction theory is the addition of electrons to the electron-deficient carbon
atom of the carboxyl group. One more theory is decarboxylation (removal of
carbon dioxide form carboxyl group). Carboxylic acids are used to synthesize
acyl halides and acid anhydrides which are generally not target compounds. They
are used as intermediates for the synthesis esters and amides, important
derivatives from carboxylic acid in biochemistry as well as in industrial
fields. There are almost
infinite esters obtained from carboxylic
acids. Esters
are formed by removal of water from an acid and an alcohol. Carboxylic acid
esters are used as in a variety of direct and indirect applications. Lower chain
esters are used as flavouring base materials, plasticizers, solvent carriers and
coupling agents. Higher chain compounds are used as components in metalworking
fluids, surfactants, lubricants, detergents, oiling agents, emulsifiers, wetting
agents textile treatments and emollients, They are also used as intermediates
for the manufacture of a variety of target compounds. The almost infinite esters
provide a wide range of viscosity, specific gravity, vapor pressure, boiling
point, and other physical and chemical properties for the proper application
selections. Amides
are formed from the reaction of a carboxylic acids with an amine.
Carboxylic
acid's reaction to link amino acids is wide in nature to form proteins (amide), the
principal constituents of the protoplasm of all cells. Polyamide is a polymer
containing repeated amide groups such as various kinds of nylon and
polyacrylamides. Carboxylic acid
are in our lives.
Caproic acid, Caprylic acid, and Capric acid containing the 6-, 8-, and 10-carbon acids respectively are a member of the series of fatty acids found in oils and animal fats. The names of Caproic, Caprylic, and Capric acids are all derived from the word caper (Latin: 'goat'). These are colorless light yellowish transparent oily liquids with unconfortable smells. These are used in organic synthesis, manufacture of perfume, medicine, lubricating grease, rubber and dye. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PHYSICAL STATE | clear liquid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MELTING POINT | 16 - 17 C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BOILING POINT |
237 - 240 C |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SPECIFIC GRAVITY | 0.911 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SOLUBILITY IN WATER | Insoluble (soluble in alcohol and ether) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AUTOIGNITION |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| pH |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VAPOR DENSITY | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NFPA RATINGS | Health: 3; Flammability: 0; Reactivity: 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
REFRACTIVE INDEX |
1.428 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FLASH POINT |
130 C |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| STABILITY | Stable under ordinary conditions. Light sensitive. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
APPLICATIONS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Organic Synthesis, Manufacture Of Perfume, Medicine, Lubricating, Grease, Rubber And Dye. Rubber & Latex, Plastics, Greases & Lubricants, Food Additives, Pharmaceuticals |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SALES SPECIFICATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
APPEARANCE |
clear to light yellow liquid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CONTENT |
99.0% min (C8) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
TITER |
15 - 18 C |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
ACID VALUE |
383 - 390 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IODINE VALUE |
0.5 max |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAPONIFICATION VALUE |
384 - 391 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| COLOR |
3Y 0.3R (5.25" Lovibond Cell) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| COLOR, APHA |
60 max |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| WATER |
0.5% max |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TRANSPORTATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PACKING | 180kgs in drum | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HAZARD CLASS | 8 (Packing Group: III) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UN NO. | 3265 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OTHER INFORMATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Symbols: C, Risk Phrases: 34, Safety Phrases: 25-33/37/39-45 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||