PRODUCT IDENTIFICATION
208-011-4
2917.19.7050
TOXICITY
SMILES
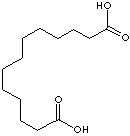
C(CCCCCCCCCCCC(O)=O)(O)=O
CLASSIFICATION
Dicarboxylic acid
PHYSICAL AND CHEMICAL PROPERTIES
245 C
NFPA RATINGS
REFRACTIVE INDEX
GENERAL DESCRIPTION & EXTERNAL LINKS
|
|
|
|
Synthesis and characterization of novel polyamides based on tridecanedioic acid: Nylons 3 13, 5 13, 6 13, 7 13, 9 13, 10 13, 11 13......... Linear polyamides with high aliphatic content were prepared through step-heating melt polycondensation of tridecanedioic acid with various diamines. The synthesized polyamides were characterized comprehensively by means of IR, NMR and Raman spectroscopy. In addition, thermogravimetry, differential scanning calorimetry and dynamic mechanical analysis were used to investigate thermal properties of the obtained polyamides. It was found that melting and crystallization temperatures decrease as the aliphatic content increases. X-ray diffraction was applied to determine the crystal structures of the polyamides........(http://www.e-polymers.org/)
APPEARANCE
98.0% min
WATER INSOLUBLES
0.5% max
- Plasticizer for polymers
- Biodegradable solvents and lubricants
- Engineering plastics
- Epoxy curing agent
- Adhesive and powder coating
- Corrosion inhibitor
- Perfumery and pharmaceutical
- Electrolyte
There are almost infinite esters obtained from carboxylic acids. Esters are formed by removal of water from an acid and an alcohol. Carboxylic acid esters are used as in a variety of direct and indirect applications. Lower chain esters are used as flavouring base materials, plasticizers, solvent carriers and coupling agents. Higher chain compounds are used as components in metalworking fluids, surfactants, lubricants, detergents, oiling agents, emulsifiers, wetting agents textile treatments and emollients, They are also used as intermediates for the manufacture of a variety of target compounds. The almost infinite esters provide a wide range of viscosity, specific gravity, vapor pressure, boiling point, and other physical and chemical properties for the proper application selections.
|
C length (Straight) |
Product |
CAS # |
Melting Point |
Boiling Point |
|
C 2 |
Oxalic
Acid (Ethanedioic Acid) |
144-62-7 |
189 - 191 C |
Sublimes |
|
C 3 |
Malonic
Acid |
141-82-2 | 131 - 135 C |
Decomposes |
|
C 4 |
Succinic
Acid |
110-15-6 |
185 - 190 C |
235 C |
|
C 5 |
Glutaric
Acid |
110-94-1 |
95 - 99 C |
302 C |
|
C 6 |
Adipic
Acid |
124-04-9 |
151 - 153 C |
265 C at 100 mmHg |
|
C 7 |
Pimelic
Acid |
111-16-0 |
105 - 106 C |
212 C at 10 mmHg |
|
C 8 |
Suberic
Acid |
505-48-6 |
143 - 144 C |
230 C at 15 mmHg |
|
C 9 |
Azelaic
Acid |
123-99-9 |
100 - 103 C |
237 C at 15 mmHg |
|
C 10 |
Sebacic
Acid |
111-20-6 |
131 - 134 C |
294 at 100 mmHg |
|
C 11 |
Undecanedioic acid | 1852-04-6 |
109 - 110 C |
|
|
C 12 |
Dodecanedioic acid | 693-23-2 |
128 - 129 C |
245 C at 10 mmHg |
|
C 13 |
Brassylic acid (Tridecanedioic acid) |
505-52-2 |
112 - 114 C |
|
|
C 14 |
Tetradecanedioic acid | 821-38-5 |
126 - 128 C |
|
|
C 15 |
Pentadecanedioic acid | 1460-18-0 |
|
|
|
C 16 |
Thapsic acid (Hexadecanedioic acid) |
505-54-4 |
124 - 126 C |
|
|
C 18 |
Octadecanedioic acid |
871-70-5 |
|
|