|
BENZOIC ACID
| ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. |
65-85-0 |
|
| EINECS NO. | 200-618-2 | |
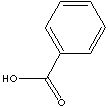
| FORMULA |
C6H5COOH | |
| MOL WT. | 122.12 | |
| H.S. CODE | 2916.31.1105 | |
|
TOXICITY |
Oral rat LD50: 1700 mg/kg | |
| SYNONYMS | Benzenemethanoic acid; Carboxybenzene; | |
| Acide benzoique (French); Acido benzoico; Benzenecarboxylic acid; Benzeneformic acid; Benzoate; Benzoesaeure; Carboxybenzene; Dracylic acid; Flowers of benjamin; Flowers of benzoin; Phenylcarboxylic acid; Phenylformic acid; Salvo liquid; Salvo powder; Benzoesäure (German); ácido benzoico (Spanish); Acide benzoïque (French); Kyselina benzoova (Czech); Dracylic acid; Other RN: 8013-63-6, 331473-08-6 | ||
|
SMILES |
c1(ccccc1)C(=O)O |
|
|
CLASSIFICATION |
Carbocyclic carboxylic acid, Preservative, Antifungal agent |
|
|
EXTRA NOTES |
A fungistatic compound that is widely used as a food preservative. It is conjugated to glycine in the liver and excreted as hippuric acid. | |
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
white crystalline flakes | |
| MELTING POINT | 122 C | |
| BOILING POINT |
249 C | |
| SPECIFIC GRAVITY | 1.321 | |
| SOLUBILITY IN WATER | 0.29 g/100 ml | |
| SOLVENT SOLUBILITY | Soluble in alcohol, benzene, ether | |
| pH | 2.8 (saturated solution at 25 C) | |
| VAPOR DENSITY | 4.2 | |
|
AUTOIGNITION |
571 C | |
|
REFRACTIVE INDEX |
| |
|
NFPA RATINGS |
Health: 2; Flammability: 1; Reactivity: 0 | |
| FLASH POINT |
121 C | |
| STABILITY | Stable under ordinary conditions | |
|
EXTERNAL LINKS & GENERAL DESCRIPTION |
||
|
Drug Information Portal (U.S. National Library of Medicine) - Benzoic acid PubChem Compound Summary - Benzoic acid IPCS INCHEM - Benzoic acid Drug Bank - Benzoic acid KEGG (Kyoto Encyclopedia of Genes and Genomes) - Benzoic acid http://www.ebi.ac.uk/chebi/ - Benzoic acid http://www.ncbi.nlm.nih.gov/ - Benzoic acid Human Metabolome Database - Benzoic acid http://www.inchem.org/ Local: |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
White, crystalline flakes | |
| ASSAY |
99.5% min | |
| MOISTURE |
0.1% max | |
| COLOR, APHA |
50 max | |
|
MELTING POINT |
121.5 C min | |
|
RESIDUE ON IGNITION |
0.1% max | |
| TRANSPORTATION | ||
| PACKING |
| |
| HAZARD CLASS | ||
| UN NO. | ||
| SAFETY INFORMATION | ||
|
HAZARD OVERVIEW |
OSHA Hazards: Harmful by ingestion., Irritant, Harmful by inhalation. |
|
|
GHS |
|
|
| SIGNAL WORD |
Danger |
|
|
PICTOGRAMS |
|
|
|
HAZARD STATEMENTS |
H318-H335 |
|
|
P STATEMENTS |
P261-P280-P305 + P351 + P338 |
|
| EC DIRECTIVES |
|
|
| HAZARD CODES |
|
|
|
RISK PHRASES |
22-36 |
|
|
SAFETY PHRASES |
26 |
|
|
PRICE INFORMATION |
||