|
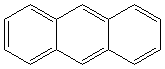
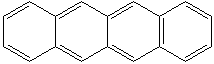
ANTHRACENE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PRODUCT IDENTIFICATION |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS NO. | 120-12-7 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EINECS NO. | 204-371-1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FORMULA | C14H10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MOL WT. | 178.23 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| H.S. CODE | 2902.90 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
TOXICITY |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SYNONYMS | Paranaphthalene; Anthracen (German); Anthraxcene; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | coal tar | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CLASSIFICATION |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PHYSICAL STATE | White to yellow crystalline flakes with green fluorescence | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MELTING POINT | 216 - 218 C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BOILING POINT | 340 C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SPECIFIC GRAVITY | 1.24 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SOLUBILITY IN WATER | Insoluble | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SOLVENT SOLUBILITY |
soluble in alcohols, benzene, Hydronaphthalenes, carbon disulfide, chloroform, and other organic solvents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AUTOIGNITION |
538 C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| pH |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VAPOR DENSITY | 6.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NFPA RATINGS | Health: 1 Flammability: 1 Reactivity: 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FLASH POINT |
121 C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| STABILITY | Stable under ordinary conditions. Light sensitive. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
APPLICATIONS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
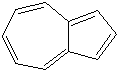
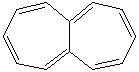
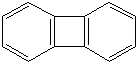
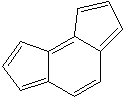
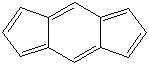
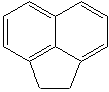
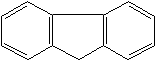
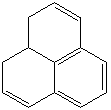
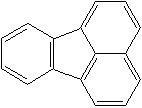
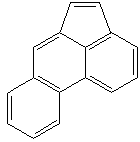
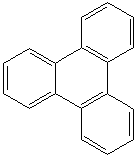
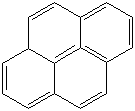
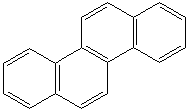
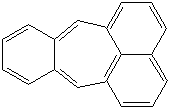
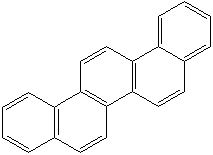
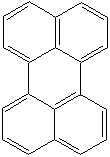
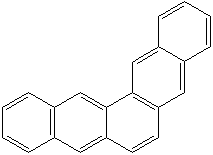
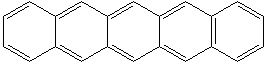
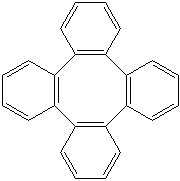
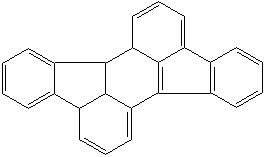
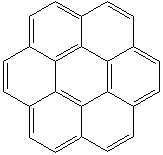
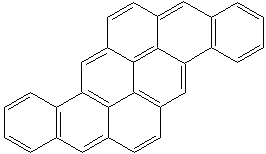
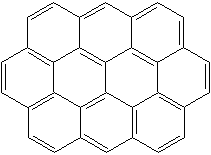
Anthracene is a tricyclic aromatic hydrocarbonn derived from coal tar; melts at 218°C,boils at 354°C, insoluble in water but is soluble in most organic solvents such as carbon disulfide, alcohols, benzene, chloroform, and hydronaphthalenes. Its molecular structure consists of three benzenelike rings joined side by side (the general formula CnH2n-18) and its oxidation yields anthraquinone, the parent substance of a large class of dyes and pigments. Anthracene is a basic substance for production of anthraquinone, dyes, pigments, insecticides, wood preservatives and coating materials. Anthracene is a nucleus for polymer soluble pigments. Anthracene forms reversible photodimer through the 9-,10- positions in response to light and provides photochromic applications. Anthracene family compounds are base materials for colorings. They have useful functions such as light and temperature sensitivity, heat resistance, conductivity, emittability, and corrosion resistance. Due to pi-electron cloud overlaps, anthracene exhibits semiconductor property. Organic semiconductors have some merits of self radiation, flexibility, light weight, easy fabrication, and low cost. They have been investigated as organic electroluminescence materials for the applications in organic solar cells, biosensitizers and display devices such as OLED(Organic Light Emiting Diode), OTFT(Organic Thin Film Transistor), Wearable Display, and e-paper. Some examples of organic electroluminescence materials are:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SALES SPECIFICATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
APPEARANCE |
yellowish flakes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ASSAY |
95.0% min | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VOLATILE CONTENT | 0.5% max | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TRANSPORTATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PACKING | 25ks in bag. 1mt in bag | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HAZARD CLASS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UN NO. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OTHER INFORMATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 22/36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
GENERAL DESCRIPTION OF PAHs |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Polycyclic aromatic hydrocarbons (also called polynuclear hydrocarbons) have two
or more single or fused aromatic rings if a pair of carbon atoms is shared
between rings in their molecules. In particular, the term 'PAH' refers to the
compounds consisting of only carbon and hydrogen atoms while the wider term
'polycyclic aromatic compounds' includes the alkyl-substituted derivatives and
functional derivatives such as nitro- and hydroxy-PAH as well as the
heterocyclic analogues, which contain one or more hetero atoms in the aromatic
structure. PAHs exist in various combinations that manifest various functions
such as light sensitivity, heat resistance, conductivity, emittability,
corrosion resistance and physiological action. The simplest examples are
naphthalene having two benzene rings side by side and biphenyl having two
bond-connected benzene rings. PAHs are not found in synthetic products and
are non-essential for the growth of living cells. The general
characteristics of PAH describe high melting- and boiling-points (they are
solid), low vapour pressure, and very low water solubility, decreasing with
increasing molecular weight whereas resistance to oxidation, reduction, and
vapourization increases. Vapour pressure tends to decrease with increasing
molecular weight. PAHs are highly lipophilic and readily soluble in organic
solvents. The lower molecular weight PAHs of 2 or 3 ring groups such as
naphthalenes, fluorenes, phenanthrenes, and anthracenes have toxicity which
tends to decrease with increasing molecular weight. PAHs are not synthesized
chemically for industrial purposes but are isolated from concentrated coal-tar
products (or from pyrolysis of coal hydrocarbons) followed by subsequent
purification through repeated distillation and crystallization. Some PAHs such
as naphthalene are also obtained from the concentration of the high boiling
residual oil (and asphalt) derived from crude petroleum refinery processing.
These PAHs are mostly used as intermediaries in pharmaceuticals, agricultural
products,
photographic products, thermosetting plastics, lubricating materials, and other
chemical
industries. General uses are;
Precise PAHs, specific refined products are used also in the field of electronics, functional plastics and liquid crystals. Pharmaceutical and agricultural PAHs obtained from coal tar are such materials as indole, indolizine, indene, quinoline, quinalidine, isoquinoline and their derivatives. High boiling-point special solvent are such materials as tetoralin, decaline, methyl-naphthalenes. Coumarins and dihydrocoumarins which can be obtained from coal tar are PAHs used in perfumery. Thermosensitive paper sensitizer PAHs are such materials as p-benzylbiphenyl and ethylbiphenyl.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||