|
SULFURIC
ACID
| ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 7664-93-9 |
|
| EINECS NO. | 231-639-5 | |
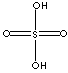
| FORMULA | H2SO4 | |
| MOL WT. | 98.08 | |
|
H.S. CODE |
2807.00 | |
| TOXICITY | Oral rat LD50: 2140 mg/kg | |
| SYNONYMS | Oil Of Vitriol; Babcock Acid; Sulphuric Acid; Battery Acid | |
| Acide Sulfurique (French); Acido Solforico (Italian); Acido Sulfurico (Spanish); Dihydrogen Sulfate; Dipping Acid; Electrolyte Acid; Hydrogen Sulfate; Mattling Acid; Schwefelsaeureloesungen; Spirit Of Sulfur; Zwavelzuuroplossingen | ||
| RAW MATERIALS | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | Colorless (pure) to dark brown, oily, dense liquid with acrid odor. | |
| MELTING POINT | 3 C (100%), -32 C (93%), -38 C (78%), -64 C (65%) | |
| BOILING POINT | ca. 290C (decomposes at 340 C) | |
| SPECIFIC GRAVITY |
1.84 | |
| SOLUBILITY IN WATER | Miscible, liberates much heat | |
| pH | 1 N solution (ca. 5% w/w) = 0.3; 0.1 N solution (ca. 0.5% w/w) = 1.2; 0.01 N solution (ca. 0.05% w/w) = 2.1 | |
| VAPOR DENSITY | 3.4 | |
|
REFRACTIVE INDEX |
| |
|
NFPA RATINGS |
Health: 3 Flammability: 0 Reactivity: 2 Other: Water reactive | |
|
AUTOIGNITION |
| |
| FLASH POINT |
| |
| STABILITY | Stable under ordinary conditions | |
|
APPLICATIONS |
||
|
It is used in fertilizers, chemicals, dyes, petroleum refining, etching and in making iron, steel and industrial explosives. |
||
| SALES SPECIFICATION | ||
|
70% , 93%, 96%, 98% SULFURIC ACID |
||
| H2SO4 | 70.0±1 % or 93.0 % min or 96.0±1 % or 98.0% min | |
|
FREE SO2 |
0.01% max | |
|
Fe |
0.01% max | |
|
OLEUM |
||
| FREE SO3 |
25% | |
|
ASH |
0.05% max | |
|
Fe |
0.03% max | |
|
LIQUID SO3 |
||
|
SO3 (DRY BASIS) |
99.9% min | |
|
MOISTURE |
0.01% max | |
|
ASH |
0.05% max | |
|
Fe |
0.03% max | |
| SULFURIC ACID MONOHYDRATE | ||
| CONTENT | 99-101 wt.% | |
| TRANSPORTATION | ||
| PACKING | ||
| HAZARD CLASS | 8 | |
| UN NO. | 1830, UN 1831 (fuming), UN 1832 (spent) | |
| GENERAL
DESCRIPTION OF SULFURIC ACID & ITS SALTS
|
||
|
Pure sulfuric acid with a specific gravity of 1.85. freezes at 10.37° C , boils at 340° C and is soluble in all proportions in water. When heated, the pure acid partially decomposes into water and sulfur trioxide. Sulfuric acid is a very strong acid in aqueous solution; it is largely changed to hydrogen ions (H+) and sulfate ions (SO42-). Each molecule gives two H+ ions, thus sulfuric acid is dibasic, which forms both normal sulfates (with both hydrogens replaced, e.g., sodium sulfate, Na2SO4) and acid sulfates, also called bisulfates or hydrogen sulfates (with only one hydrogen replaced, e.g., sodium bisulfate, NaHSO4). Dilute solutions of sulfuric acid show all the behavior characteristics of acids. It turns blue litmus red. It conducts electricity, neutralizes alkalies, corrodes active many metals, releasing hydrogen gas, and forming the sulfates. It reacts with most hydroxides and oxides, with some carbonates and sulfides, and with some salts. Concentrated sulfuric acid, formerly called oil of vitriol, is a weak acid and a poor electrolyte because relatively little of it is dissociated into ions. When concentrated sulfuric acid is heated, it behaves also as an oxidizing agent dissolving relatively unreactive metals as copper, mercury, and lead to produce metal sulfate, sulfur dioxide, and water. Because the concentrated acid has a fairly high boiling point, it can be used to release more volatile acids from their salts, or common salts, when are heated with concentrated sulfuric acid, HCl gas is evolved. As concentrated sulfuric acid has a very strong affinity for water, it is a valuable desiccating agent, is used as a drying agent and can be used to dehydrate many compounds. It removes water from, and therefore chars, wood, cotton, sugar, and paper. It is used in the manufacture of ether, nitroglycerin, and dyes for its property as a desiccant. Sulfur trioxide dissolves readily in concentrated sulfuric acid to form pyrosulfuric acid, H2S2O7, which is also called fuming sulfuric acid or oleum. Sulfuric acid is prepared industrially by the reaction of water with sulfur trioxide, which in turn is made by chemical combination of sulfur dioxide and oxygen either by the contact process or the chamber process. The lead chamber process is used to produce much of the acid used to make fertilizers. It produces a relatively dilute acid (62% - 78%). The contact process produces a more concentrated acid but requires purer raw materials and the use of expensive catalysts. Some sulfuric acid is also made from ferrous sulfate waste solutions from pickling iron and steel and from waste acid sludge from oil refineries. The uses of sulfuric acid are so varied that the volume of its production provides an approximate index of general industrial activity. Its main use is in phosphate fertilizer production, both superphosphate of lime and ammonium sulfate. It is widely also used to manufacture chemicals, e.g., in making hydrochloric acid, nitric acid, sulfate salts, synthetic detergents, dyes and pigments, explosives, drugs, other acids, parchment paper, glue and wood preservatives. It is used in the purification of petroleum to wash impurities out of gasoline and other refinery products.Sulfuric acid is used in processing metals, e.g., in pickling (cleaning) of metal, electroplating baths, nonferrous metallurgy. Rayon is made with sulfuric acid. In one of its most familiar applications, it serves as the electrolyte in the lead-acid storage battery commonly used in motor vehicles (acid for this use, containing about 33% H2SO4 and with specific gravity about 1.25, is often called battery acid). Any of numerous
chemical compounds related to sulfuric acid,
formed by replacing one or both of the hydrogens with a metal or
a radical are called sulfates ( also spelled sulphates). Sulfates
are salts or esters of sulfuric acid. One
group of these derivatives is composed of salts containing the sulfate ion, and positively charged ions such as those of
sodium, magnesium, or ammonium; a second group is composed of esters, in which
the hydrogen atoms of sulfuric acid have been replaced by carbon-containing
combining groups such as methyl (CH3) or ethyl
(C2H5). Most metal
sulfates are readily soluble in water, but calcium and mercuric sulfates are
only slightly soluble, while barium, lead, strontium, and mercurous sulfates are
insoluble. In chemical analysis, the sulfate ion,
SO42-, is
usually detected by adding barium chloride solution; the white barium sulfate
precipitate that forms is insoluble in hydrochloric acid. Sulfates are widely
distributed in nature. Barium sulfate occurs as barite; calcium sulfate is found as gypsum, alabaster, and selenite; Epsom salts is magnesium sulfate; sodium sulfate occurs as its decahydrate, Glauber
salt; and strontium sulfate occurs as
celestite. Some sulfates were formerly known
as vitriols; blue vitriol is cupric sulfate,
green vitriol is ferrous sulfate, and white
vitriol is zinc sulfate. Alums are solid sulfates, containing two different
metals and two sulfate radicals. Organic sulfates are esters. They can be formed
by reacting an alcohol with cold sulfuric acid. They are also formed by the
reaction of sulfuric acid with a solid bond in an alkene; the product is called
an alkyl hydrogen sulfate. An alkyl hydrogen sulfate can be broken down to an
alcohol and sulfuric acid by heating it with water (hydrolysis); this reaction is often used to
synthesize alcohols.
|
||