| SODIUM CYANOBOROHYDRIDE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 25895-60-7 |
|
| EINECS NO. | 247-317-2 | |
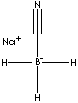
| FORMULA | NaBH3CN | |
| MOL WT. | 62.84 | |
| H.S. CODE | 2850.00 | |
|
TOXICITY |
||
| SYNONYMS | Sodium cyanotrihydridoborate; | |
| (cyano-C)trihydro-Borate(1-), sodium; Sodium cyanotrihydridoborate; Sodium cyanotrihydroborate; | ||
|
SMILES |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | white crystalline powder | |
| MELTING POINT | 241 - 243 C | |
| BOILING POINT | ||
| SPECIFIC GRAVITY |
1.20 | |
| SOLUBILITY IN WATER | Reacts | |
| pH |
| |
| VAPOR DENSITY | ||
| AUTOIGNITION |
| |
| NFPA RATINGS | Health: 4; Flammability: 3; Reactivity: 2 | |
|
REFRACTIVE INDEX |
| |
| FLASH POINT |
| |
| STABILITY | Hygroscopic (dangerous when wet). | |
|
APPLICATIONS |
||
|
Sodium borohydride is a milder reducing agent, can be used in aqueous solution. It converts selectively aldehydes and ketones the corresponding alcohols in the manufacture of pharmaceuticals and other fine chemicals. It will not react with esters, amides, or carboxylic acids, the more powerful reducing agent lithium aluminum hydride(LAH) is used to reduce these compounds. LAH is the more powerful reducing agent than sodium borohydride due to the weaker Al-H bond compared to the B-H bond. The reactivity of sodium borohydride can be modified by addition of iodine or methanol in BH3-THF to reduce esters into the corresponding alcohols like the reaction of benzyl benzoate to benzyl alcohol. Sodium borohydride is used as a hydrogen source for fuel cell systems and a foaming agent for rubbers. Sodium cyanoborohydride converts certain alcohol groups to methylene groups. Sodium Cyanoborohydride is used as a selective amination reductant. It converts aldehydes (chemoselective), ketones (stereoselective) to the corresponding alcohols in the manufacture of pharmaceuticals and other fine chemicals. It is used in the reductive alkylation of amines, novel metals and oximes. Some examples of reducing agents are:
|
||
| SALES SPECIFICATION | ||
| APPEARANCE |
white crystalline powder | |
| CONTENT |
95.0% min | |
|
WATER |
0.1% max | |
| TRANSPORTATION | ||
| PACKING | 5kgs in drum | |
| HAZARD CLASS | 4.3 (Packing Group: II) | |
| UN NO. | 3132 | |
| OTHER INFORMATION | ||
| Hazard Symbols: T F C, Risk Phrases: 10-15-23/24/25-32-34, Safety Phrases: 7-8-26-36/37/39-45 | ||
| GENERAL DESCRIPTION OF HYDRIDE | ||
Hydride is the isolated atomic hydrogen anion, H- or any compound containing
hydrogen and another element, more electropositive element or group. Hydride
consists of a singly charged positive nucleus and two electrons of which one
electron is weakly held and readily donative ��extra��. There are some types of hydrides according to their bonding.
Hydrides which carry hydrogen can provide large amounts of heat when burned. They can be used as a component in jet fuels. They are less flammable and less volatile than hydrocarbon fuels. They are relatively environmentally friendly because they degrade quickly in the environment. Hydrides and hydrido complexes containing this easily polarized ion are highly reactive, strongly basic and powerfully reducing in synthetic reactions. They are important reducing agents in industrial reactions though they are easily destroyed in the relatively acidic compound water (H2O) and in air containing dioxygen (O2). Examples of commercially useful hydride complexes are:
|
||