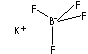| POTASSIUM TETRAFLUOROBORATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. |
14075-53-7; 1303-68-0; 12692-95-4; 12712-39-9 |
|
| EINECS NO. | 237-928-2 | |
| FORMULA |
KBF4 |
|
| MOL WT. |
125.89 |
|
| H.S. CODE | 2826.90.9000 | |
|
TOXICITY |
Rat LD50 (intraperitoneal): 240mg/kg | |
| SYNONYMS | Potassium Fluoborate; Potassium Borofluoride; Avogadrite; |
|
|
Borate(1-), tetrafluoro-, potassium; Potassium boride fluoride; Potassium fluoroborate; Kaliumtetrafluoroborat (German); Tetrafluoroborato de potasio (Spanish); Tétrafluoroborate de potassium (French); |
||
| SMILES |
[B-](F)(F)(F)F.[K+] |
|
|
CLASSIFICATION |
Tetrafluoroborate, Hydrogen compound, Acid |
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
white crystalline powder |
|
| MELTING POINT |
530 (Decomposes) |
|
| BOILING POINT | ||
| SPECIFIC GRAVITY |
2.5 |
|
| SOLUBILITY IN WATER | ||
| pH | ||
| VAPOR DENSITY | ||
| AUTOIGNITION |
|
|
| NFPA RATINGS | ||
|
REFRACTIVE INDEX |
||
| FLASH POINT | Not considered to be a fire hazard | |
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & EXTERNAL LINKS |
||
|
Fluoroboric acid is used in plating circuits; metal finishing;
electropolishing of aluminium
and its alloy; component of galvanic baths; organic synthesis as catalyst for
alkylations and polymerisation; stabilisation of diazo salts; manufacturing of
inorganic fluoroborate salts.
Inorganic fluoroborate salts are used as components of fluxing and plating, as catalysts, in flame-retardant manufacture, in metal treatment; grain refining agents; as active fillers in resin bonded abrasives; in electrolytic generation of boron, preparation of glazing frits. Wikipedia Linking: http://en.wikipedia.org/wiki/Tetrafluoroborate http://www.solvaychemicals.com/ http://molecules.gnu-darwin.org/mod/Potassium-more.html http://www.faqs.org/patents/app/20090139607 |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white crystalline powder |
|
|
KBF4 |
98.0% min |
|
|
Fe2O3 |
0.01% max |
|
|
SO4 |
0.02% max |
|
|
SiO2 |
0.5% max |
|
|
Cl |
0.1% max |
|
|
HEAVY METAL |
5ppm max |
|
|
WATER |
0.1% max |
|
| TRANSPORTATION | ||
| PACKING |
50kg in bag |
|
| HAZARD CLASS | 8 (Packing group:II) | |
| UN NO. | 1759 | |
| OTHER INFORMATION | ||
| Hazard Symbols: C, Risk Phrases: 34, Safety Phrases: 26-36/37/38-45 |
||
| GENERAL DESCRIPTION OF BORIC ACID |
||
| Boric acid refers to 3 compounds; orthoboric acid (also called boracic acid, H3BO3 or B2O3·3H2O), metaboric acid (HBO2 or B2O3·H2O), and tetraboric acid (also called pyroboric, H4B4O7 or B2O3·H2O). Orthoboric acid dehydrates to form metaboric acid and tetraboric acid above 170 C and 300C respectively. Orthoboric acid is derived from boric oxide in the form of white, triclinic crystals. It is poorly soluble in cold water but dissolves readily in hot water, in alcohol and glycerine. Metaboric acid is a white, cubic crystalls. It is soluble in water slightly. Tetraboric acid is a white solid soluble in water. When tetraboric and metaboric acid are dissolved, it reverts to orthoboric acid. The main uses of boric acid is to make borate salts such as borax and other boron compounds. Boric acid is also used in heat resistant glass, in fireproofing fabrics, in electroplating baths, in leather manufacturibg, porcelain enamels and in hardening steels. Boric acid has antiseptic and antiviral activity. Aqueous solutions have been used as mouth-washes, eye-drops, skin lotions and cosmetics. Boric acid and its salts are components of many commerical insecticides and wood preservatives. | ||