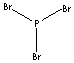| PHOSPHORUS TRIBROMIDE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 7789-60-8 |
|
| EINECS NO. | 232-178-2 | |
| FORMULA | PBr3 | |
| MOL WT. | 270.69 | |
|
H.S.CODE |
2812.90 | |
| TOXICITY | ||
| SYNONYMS | Phosphorus (III) bromide; Tribromophosphine; | |
| Phosphoric (III) bromide; | ||
| SMILES | phosphorus with bromide | |
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | colourless fuming liquid with irritating odor | |
| MELTING POINT | -40 C | |
| BOILING POINT | 175 C | |
| SPECIFIC GRAVITY | 2.88 | |
| SOLUBILITY IN WATER | decomposes | |
| pH | ||
| VISCOSITY | ||
| VAPOR DENSITY | 9.3 | |
|
AUTOIGNITION |
| |
|
NFPA RATINGS |
Health: 3 Flammability: 0 Reactivity: 2 Other: water reactive | |
|
REFRACTIVE INDEX |
||
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
| Phosphorus Tribromide is a colourless fuming liquid with irritating odor; specific gravity 2.88; melting point -40 C; boiling point 175 C. The liquid reacts with water rapidly to produce hydrogen bromide and phosphoric acids. It is prepared by direct union of phosphorus (not excess) with bromine vapour. (excess contaminates the product with phosphorus(V) bromide). The crude is then purified by distillation. It reacts readily with hydroxyl groups, which acts as a bromine atoms provider in many organic chemical synthesis. It is known that Phosphorus Tribromide is used to prepare pharmaceuticals such as Alprazolam, Fenoprofen, Methohexital. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
colorless to pale yellow liquid | |
|
ASSAY |
99.0% min |
|
|
DISTILLATION RANGE |
171 - 174 C |
|
| SPECIFIC GRAVITY |
2.85 - 2.89 |
|
|
TRANSPORTATION |
||
| PACKING |
| |
| HAZARD CLASS | 8 (Packing Group: II) | |
| UN NO. | 1808 | |
| OTHER INFORMATION | ||
| European Hazard Symbols: C, Risk Phrases: 14-34-37, Safety Phrases: 26-45 | ||
| DESCRIPTION OF PHOSPHORUS | ||
| Phosphorus is a nonmetallic chemical element in group 15 (nitrogen family, formerly Va) of periodic table; atomic number 15 atomic mass 30.9738; melting point ca 44.1 C (white); boiling point ca 280 C (white); specific gravity 1.82 (white), 2.34 (red), 2.70 (black); valence -3, +3, or +5 ; electronic config. 2-8-5 or 1s 22s 22p 63s 23p 3. The phosphorus molecule is composed of four phosphorus atoms, P4. Phosphorus exists in a number of allotropic forms [white (alpha and beta), red, black and/or violet] in the same physical state. White phosphorus is a white to yellow waxy substance which ignites spontaneously in air to form white fumes of phosphorus pentoxide and glows without emitting heat. Phosphorus is stored underwater as it is extremely poisonous, insoluble in water (but soluble in carbon disulfide). Commercial production of elemental phosphorus is prepared from phosphorite or phosphate rock (apatite, an impure calcium phosphate mineral) reacting with coke and sand or silica pebblesor at high temperatures in an electric furnace. Calcium silicate is produced as a by-product. White phosphorus is used as a deoxidizing agent in the preparation of steel and phosphor bronze. It is also used in rat poisons and to make smoke screens (by burning) for warfare. When white phosphorus is heated to about 250 C with air absence, it changes into the red phosphorus. Red phosphorus, a dark redish powder or crystal, does not ignite spontaneously unless heated to 200 C, does not phosphoresce and it is a little less dangerous than white phosphorus. It is used to make matches. Red phosphorus is prepared commercially by heating calcium phosphate with sand and coke in an electric furnace. Black allotrope is obtained industrially by heating at 300 C under pressure with a mercury catalyst. It has a layer structure and is stable. The major use of phosphorus compounds is in fertilizers, mainly as a mixture called superphosphate (calcium hydrogen phosphate), obtained from phosphate minerals by sulfuric acid treatment; and in nitrophosphates. Phosphorus is burned to make phosphorus pentoxide [phosphorus(V) oxide], a white solid used as a chlorinating agent in organic chemistry, as a drying agent and mainly converted to phosphoric acid used to make phosphates for fertilizers, electro chemical polishing and shaping, electroplating, metal cleaning and pickling in metal treatment by reaction with water. Phosphorus is highly reactive. A wide range of compounds is formed for uses in detergents, water softeners, pharmaceuticals, dentifrices, and in many other important applications. It forms metal phosphides and covalently bonded phosphorus(III) and phosphorus(V) compounds. Phosphoric acid can combine with certain alkaline elements to form salts called phosphates. | ||