PRODUCT IDENTIFICATION
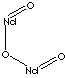
HS CODE
CLASSIFICATION
Neodymium compound
PHYSICAL AND CHEMICAL PROPERTIES
2270 C
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
USA.gov - Neodymium oxide
Wikipedia Linking - Neodymium(III) oxide
Google Scholar Search - Neodymium oxide
U.S. National Library of Medicine - Neodymium oxide
PubChem Compound Summary - Neodymium oxide
NCBI (http://www.ncbi.nlm.nih.gov/) - Neodymium oxide
Material Safety Data Sheet - Neodymium oxide
EPA - Substance Registry Services - Neodymium oxide
Local:
Neodymium is a lustrous, silver-yellow, rare-earth metallic element in the lanthanide series of group IIIb of the periodic table. Symbol Nd; Aomic number 60; atomic mass 144.24; melting point ca 1,021°C; boiling point ca 3,068°C; specific gravity 6.80 or 7.004; valence +3; electronic config. [Xe]4f46s². Neodymium tarnishes in air, forming an oxide form that splits and exposes to further oxidation. The coated form does not protect the metal from further oxidation (it must be stored away from contact with air). The metal is also attacked by water and by acids. Thought it has a hexagonal crystalline structure, but it assumes a face-centered cubic conformation with specific gravity about 6.8 when heated above 800°C. Neodymium is not found in nature as the free element but in the minerals monazite and bastnasite. The metal may be prepared from electrolysis of its halides or through an ion exchange process. Neodymium is used in coloring glasses and for doping some glass lasers since it transmits 90% of the blue, green, and red light rays and no more than 10% of the yellow. Neodymium compounds include:
- Neodymium Acetate (CAS #: 25721-92-0, Nd(C2H3O2)3)
- Neodymium Bromide (CAS #: 13536-80-6, NdBr3)
- Neodymium Carbonate Hydrate (CAS #: 38245-38-4, Nd2(CO3)3)·XH2O)
- Neodymium Chloride (CAS #: 10024-93-8, NdCl3 or 13477-89-9 (Hexahydrate))
- Neodymium Fluoride (CAS #: 13709-42-7, NdF3)
- Neodymium Hydroxide Hydrate (16469-17-3, Nd(OH)3·XH2O)
- Neodymium Iodide (CAS #: 13813-24-6, NdI3)
- Neodymium Nitrate Hydrate(CAS #: 14517-29-4, Nd(NO3)3)·XH2O)
- Neodymium Nitride (NdN)
- Neodymium Oxalate Hydrate (28877-87-4, Nd2(C2O4)3·XH2O)
- Neodymium Oxide (CAS #: 1313-97-9, Nd2O3)
- Neodymium Sulfate Hydrate (101509-27-7, Nd2(SO4)3·XH2O)
- Neodymium Sulfide (Nd2S3 )
- Neodymium Telluride (CAS #: 12035-35-7, Nd2Te3)
APPEARANCE