| CHLORINATED TRISODIUM PHOSPHATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 56802-99-4, 11084-85-8 |
|
| EINECS NO. | 234-307-8 | |
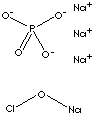
| FORMULA | (Na3PO4·11H2O)4·NaOCl | |
| MOL WT. | ||
|
H.S. CODE |
||
|
TOXICITY |
||
|
SYNONYMS |
Sodium hypochlorite phosphate; | |
| Chlorinated trisodium salt of phosphoric acid; Tridecasodium hypochlorite tetrakis (phosphate); Hipocloritotetrakis (fosfato) de tridecasodio; Hypochloritetétrakis (phosphate) de tridécasodium; | ||
| SMILES | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
|
PHYSICAL STATE |
white crystalline powder | |
| MELTING POINT | Decomposes (60 C) | |
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | Complete | |
| pH | 11.5 - 12.0 (1% solution) | |
| VAPOR DENSITY | ||
|
AUTOIGNITION |
| |
|
NFPA RATINGS |
||
|
REFRACTIVE INDEX |
| |
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions | |
|
APPLICATIONS |
||
|
Sodium hypochlorite (NaOCl), is commercially available in several solution
concentrations, with 12% being the most common for bulk, as this material is
highly unstable salt which become sodium chlorate easily. It is formed by the
electrolysis of sodium chloride solution when chlorine is bubbled through cold
caustic soda solution. Its powerful disinfection and oxidation properties offer
a wide range of applications include bleaching in both the paper and textile
industries, water purification, odour control, chemical Intermediates and etc.
Sodium hypochlorite is used in household bleach material to remove stains
particularly on cotton. Chlorine is a general biocide substance killing germs,
micro-organisms, algae, etc. The most widely used chloride chemical
disinfectants are chlorine, ozone, chlorine dioxide and chloramine. Hypochlorite
is an alternative choline source when chlorine gas is impractical. The
commercially available liquid hypochlorite form is sodium hypochlorite (NaOCl) which is used
as the disinfectant in hospitals. But this is highly caustic, ethanol class
disinfection has replaced. Sodium hypochlorite have also been used extensively
in the disinfection of drinking-water. Hypochlorite anion,ClO-, changes the oxidation-reduction potential of the cell,
and resulting in the inactivations of the micro-organism's function. Hypochlorite [or
chlorate(I)] contains the ion, ClO- ; chlorite [or chlorate(III)],
ClO2-; Chlorate [or chlorate(V)], ClO3-;
perchlorate [or chlorate(VII)], ClO4-. Hypochlorite solution gradually releases
chlorine into water. Sodium hypochlorite is used as a bleaching
and disinfection agent for both industry and household,
oxidant, sterilizer, decoloring agent, deodorant, water Treatment
amd food
additive.
Sodium hypochlorite phosphate, an inclusion complex of trisodium phosphate and sodium hypochlorite, is a dry form of sodium hypochlorite. providing germicidal and disinfectant properties as well as alkalinity. It is used in as a bactericide in food and dairy processing and cleanser of medical instruments and scouring. It is used in detergents, automatic dishwasher detergent and laundry soaps. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white crystalline powder | |
| P2O5 |
17.5% min | |
|
ACTIVE Cl |
2.6% min | |
|
ARSENIC(As) |
1ppm max |
|
|
HEAVY METAL(Pb) |
20 ppm max |
|
| pH | 11.5 - 12.0 (1% solution) | |
| TRANSPORTATION | ||
| PACKING | ||
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: XI, Risk Phrases: 31-36/38, Safety Phrases: 26 | ||