| CEROUS FLUORIDE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PRODUCT IDENTIFICATION |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS NO. | 7758-88-5 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EINECS NO. | 231-841-3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
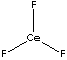
| FORMULA | CeF3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MOL WT. | 197.11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
H.S. CODE |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
TOXICITY |
Oral rat LD50: >5000 mg/kg | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
SYNONYMS |
Cerium fluoride; Cerium (III) fluoride; Cerium trifluoride; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Certrifluorid; Trifluoruro de cerio; Trifluorure de cerium; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DESCRIPTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CLASSIFICATION |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PHYSICAL STATE |
white powder | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MELTING POINT | > 1450 C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BOILING POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SPECIFIC GRAVITY | 6.16 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SOLUBILITY IN WATER | Slowly decomposes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| pH |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VAPOR DENSITY | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AUTOIGNITION |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NFPA RATINGS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| REFRACTIVE INDEX |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FLASH POINT |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| STABILITY | Stable under ordinary conditions. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
GENERAL DESCRIPTION & APPLICATIONS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Cerium is a
metallic chemical element in group IIIb of periodic table; symbol Ce, soft,
lustrous, ductile, iron-color metal with hexagonal or cubic crystalline
structure; atomic number 58; atomic mass 140.12; melting point ca 799 C; boiling
point ca 3,426 C; specific gravity 6.77 g/cm3 at 25 C; oxidation
state +3 or +4; electronic config. [Xe]4f15d16s1. It is the most
abundant rare-earth element and the most reactive next to europium among the
lanthanide group. Lanthanide (or lanthanoid) series are the group of rare-earth elements from
lanthanum (atomic
numbers 57) to 71; their chemical properties are similar to each other. The chemical properties
of scandium and yttrium are also similar to the lanthanides;
these group are often referred to "rare earth elements".
Cerium occurs chiefly in the minerals monazite and bastnaesite. The naturally occurring element is made up of the isotopes 136Ce 0.193%, 138Ce 0.250%, 140Ce 88.58%, and 142Ce 11.07% which is a radioactive alpha emitter having a half-life of 5×1015 years. Cerium is slightly harder than lead. It tarnishes rapidly in moist air but not in dry air. It oxidizes slowly in cold water and rapidly in hot water. It reacts rapidly with the solutions of alkalis and concentrated (or dilute) acids. Its oxidation valence is readily transferred form 3 to 4 if compressed or cooled. Its shell orbitals are relatively close between 4f and the outer. with Cerium of valence +3 is referred to as cerous, while with valence 4 is as ceric. Generally cerous salts are yellow to reddish while ceric slats are white. Minute particles of pure cerium or some iron-cerium alloy ignite if scratched and are used as the flint in cigarette and gas lighters. Cerium is used in making lamp mantles as incandescence is formed when burned. Cerium is allied to form malleable iron, harder stainless steel, heat resistant magnesium and aluminium alloys and permanent magnets. It is also used as an opacifier and polisher in the glass industry, in cored carbon arcs to increase the brilliance especially for the motion picture industry, and as a liquid-liquid extraction agent to remove fission products from spent uranium fuel. Cerium compounds include;
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SALES SPECIFICATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
APPEARANCE |
white to grey powder | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
REO/TREO |
99.0 - 99.99% | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TREF | 80.0% min | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TRANSPORTATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PACKING | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HAZARD CLASS | 8 (Packing Group: III) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UN NO. | 3260 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
OTHER INFORMATION |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Symbols: XI, Risk Phrases: 32-36/37/38, Safety Phrases: 22-24/25 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PRICES |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||