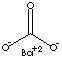| BARIUM CARBONATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 513-77-9 |
|
| EINECS NO. | 208-167-3 | |
| FORMULA | BaCO3 | |
| MOL WT. | 197.34 | |
|
HS CODE |
2836.60 | |
| TOXICITY | Oral, rat: LD50 = 418 mg/kg. | |
| SYNONYMS | Carbonic acid, barium salt (1:1) | |
| SMILES | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | White powder, poisonous | |
| MELTING POINT |
811 C |
|
| BOILING POINT | 1300 C | |
| SPECIFIC GRAVITY | 4.29 | |
| SOLUBILITY IN WATER | Almost insoluble (Soluble in acid) | |
| pH | ||
| VAPOR DENSITY | ||
|
AUTOIGNITION |
|
|
|
NFPA RATINGS |
Health: 2 Flammability: 0 Reactivity: 0 | |
|
REFRACTIVE INDEX |
|
|
| FLASH POINT | Not considered to be a fire hazard | |
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & EXTERNAL LINKS |
||
|
Wikipedia Linking: http://en.wikipedia.org/wiki/Barium The most important use of elemental barium is as a scavenger removing last traces of oxygen and other gases in television and other electronic tubes. Besides, an isotope of barium, 133Ba, is routinely used as a standard source in the calibration of gamma-ray detectors in nuclear physics studies. Barium is an important component of YBCO superconductors. An alloy of barium with nickel is used in spark plug wire. Barium oxide is used in a coating for the electrodes of fluorescent lamps, which facilitates the release of electrons. Barium compounds, and especially barite (BaSO4), are extremely important to the petroleum industry.
Manufacturing of other Barium Compounds, Glass industry, kinescope shell,, Brick Industry, Ceramics in Electrical Engineering, Fine Ceramics, Sanitary Ceramics, Chemical / Pharmaceutical Industry, Hard Ferrites, water purifying, building material. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
White amorphous Powde or Granule |
|
| BaCO3 |
99.00%
min
|
|
| MOISTURE |
0.25% max |
|
| HCl Insoluble Matter |
0.15% max |
|
|
Total Sulfur (As SO4 ) |
0.20% max |
|
| Iron (as Fe2O3 ) |
0.04% max |
|
|
Bulk Density (Powder ) |
1.48g/cm3 min (Dense) , 1.21 g/cm3 max (Light) |
|
|
Particle Size (Granule ) |
> 850micron : 1% max, 150micron >: 75% min |
|
| TRANSPORTATION | ||
| PACKING | 25kgs, 50kgs in Bag | |
| HAZARD CLASS | 6.1 (Packing Group: III) | |
| UN NO. | 1564 | |
| OTHER INFORMATION | ||
| Hazard Symbols: XN, Risk Phrases: 22, Safety Phrases: 24/25 | ||