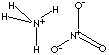
| AMMONIUM
NITRATE
|
||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. |
6484-52-2 |
|
| EINECS NO. | 229-347-8 | |
| FORMULA | NH4NO3 | |
| MOL WT. |
80.04 |
|
|
H.S. CODE |
3102.30 | |
|
TOXICITY |
Oral rat LD50: 2217 mg/kg | |
|
SYNONYMS |
Nitric acid, ammonium salt; Ammonium(I) nitrate (1:1); | |
| Ammonium saltpeter; Herco prills; Varioform; Nitrate d'ammonium (French); Nitrato amonico (Spanish); Ammoniumnitrat (German); | ||
| SMILES | ||
|
CLASSIFICATION |
|
|
|
GENERAL DESCRIPTION |
||
|
AMMONIUM NITRATE, a salt of ammonia and nitric acid, is a colourless, crystalline substance melting at 169 C. It is highly soluble in water and soluble in alcohol and liquid ammonia. Heating may cause violent combustion or explosion. Heating of the water solution or burning decomposes the salt to toxic fumes (nitrous oxide). Ammonium Nitrate reacts with combustible and reducing materials as it is a strong oxidant. It is prepared commercially by reaction of nitric acid and ammonia. It is widely used in fertilizers and explosives. The commercial grade contains about 34 percent nitrogen, all of which is in forms utilizable by plants; it is the most common nitrogenous component of artificial fertilizers. Ammonium nitrate also is employed to modify the detonation rate of other explosives, such as nitroglycerin in the so-called ammonia dynamites, or as an oxidizing agent in the ammonals. Ammonium nitrate is also used in the treatment of titanium ores and in solid-fuel rocket propellants, in pyrotechnics. |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
|
PHYSICAL STATE |
white to beige solid |
|
| MELTING POINT |
170 C |
|
| BOILING POINT |
210 C (Decomposes) |
|
| SPECIFIC GRAVITY |
1.73 |
|
| SOLUBILITY IN WATER | 118g/100g | |
| pH |
5.4 |
|
| VAPOR DENSITY | 2.8 | |
|
AUTOIGNITION |
|
|
|
NFPA RATINGS |
Health: 2 Flammability: 0 Reactivity: 3 Other: Oxidizer | |
|
REFRACTIVE INDEX |
|
|
|
FLASH POINT |
Not combustible | |
| STABILITY | Stable under ordinary conditions | |
|
APPLICATIONS |
||
| safety explosives, pyrotechnics, matches,fertilizers,herbicides and insecticides, manufacture of nitrous oxide, freezing mixtures, oxidizer in solid rocket propellants, nutrient for antibiotics and yeast, catalyst. | ||
| SALES SPECIFICATION | ||
| POROUS PRILL | ||
| CONTENT | 99.5% min | |
| N CONTENT | 34.5% min | |
| RESIDUE ON IGNITION |
0.6% max |
|
| INSOLUBLES IN WATER |
1.0% max |
|
| MOISTURE |
0.3% max |
|
| UREA as (NH2)2CO |
0.1% max |
|
| CHLORIDES as Cl |
5ppm max |
|
| NITRATES as NO2 |
1ppm max |
|
| WATER INSOLUBLE IRON |
5ppm max |
|
|
IRON |
10 ppm max |
|
|
pH |
4.2 - 6.0 (40g/I solution) |
|
| BULK DENSITY | 0.85 max (g/cm3) | |
|
PARTICLE SIZE |
1.0 - 2.4 mm (80% min) | |
| POROSITY | 15 - 25% | |
| OIL ABSORPTION |
8.0 - 15.0% |
|
| INDUSTRIAL GRADE | ||
| CONTENT | 98.5% min | |
| MOISTURE | 1.0% max | |
|
pH |
4.0 min (40g/I solution) |
|
| INSOLUBLES | 0.2% max (in 10% acid) | |
| PARTICLE SIZE |
1.0 - 3.0 mm (85%) | |
| AGRICULTURAL GRADE | ||
| CONTENT | 98.5% min | |
|
N CONTENT |
34.5% min | |
|
pH |
4.0 min (40g/I solution) |
|
| PARTICLE SIZE |
1.0 - 2.4 mm (80% min) | |
| MOISTURE |
0.6% max |
|
| AMMONIUM NITRATE LIQUID | ||
| CONTENT |
88.0% max |
|
|
pH |
4.3 - 6.0 |
|
|
TRANSPORTATION |
||
| PACKING | 50kgs in bag, 1mts in bag | |
| HAZARD CLASS | 5.1 (Packing group;III) | |
| UN NO. | 1942 | |
| GENERAL DESCRIPTION OF NITRIC ACID & ITS SALTS | ||
|
Nitric Acid is a colourless, highly corrosive, poisonous liquid (freezing point : -42° C, boiling point: 83° C) that will react with water or steam to produce heat and toxic, corrosive, and flammable vapors. It is toxic and can cause severe burns. It is an important industrial chemical for the manufacture of fertilizers, dyes, drugs, plastics, and explosives. Nitric acid is prepared commercially by the two-stage oxidation of ammonia (Ostwald process) to nitrogen dioxide. Ammonia gas is successively oxidized to nitric oxide and nitrogen dioxide by air or oxygen in the presence of a platinum gauze catalyst, which is then absorbed in water to form nitric acid. The resulting acid-in-water solution (about 50-78% by weight acid) can be dehydrated by distillation with sulfuric acid. It is miscible with water in all proportions. The nitric acid of commerce is typically a solution of 52% to 68% nitric acid in water. More concentrated solutions are available. It forms an azeotrope that has the composition 68% nitric acid and 32% water and that boils at 120.5°C. Solutions containing over 86% nitric acid are commonly called fuming nitric acid; they often have a reddish-brown color from dissolved nitrogen oxides.In aqueous solution it is both a strong acid and a powerful oxidizing agent. Among the many important reactions of nitric acid are: neutralization with ammonia to form ammonium nitrate, used widely in fertilizers and explosives; nitration of glycerol and toluene, forming the explosives nitroglycerin and trinitrotoluene, respectively; preparation of nitrocellulose; and oxidation of metals to the corresponding oxides or nitrates. Many compounds are oxidized by nitric acid. Nonmetallic elements such as carbon, iodine, phosphorus and sulfur are oxidized by concentrated nitric acid to their oxides or oxyacids with the formation of NO2 . Hydrochloric acid, aqueous HCl, is readily oxidized by concentrated nitric acid to chlorine and chlorine dioxide. The action of nitric acid on a metal usually results in reduction of the acid (i.e., a decrease in the oxidation state of the nitrogen). The products of the reaction are determined by the concentration of nitric acid, the metal involved (i.e., its reactivity), and the temperature. In most cases, a mixture of nitrogen oxides, nitrates, and other reduction products is formed. Relatively unreactive metals such as copper, silver, and lead reduce concentrated nitric acid primarily to NO2. The reaction of dilute nitric acid with copper produces NO, while more reactive metals, such as zinc and iron, react with dilute nitic acid to yield N2O. When extremely dilute nitric acid is used, either nitrogen gas or the ammonium ion (NH4+) may be formed. Nitrates, which are salts or esters of nitric acid contains NO3- (nitrate ion), are formed by replacing the hydrogen with a metal (e.g., sodium or potassium) or a radical (e.g., ammonium or ethyl). Some important inorganic nitrates are potassium nitrate (used in explosives, fireworks, matches, and fertilizers, and as a preservative in foods. It is sometimes used in medicine as a diuretic.), sodium nitrate (used in making potassium nitrate, fertilizers, and explosives.), silver nitrate (used in the preparation of silver salts for photography, in chemical analysis, in silver plating, in inks and hair dyes, and to silver mirrors.), and ammonium nitrate (Major uses are in fertilizers and explosives). Calcium nitrate is used in fertilizers; barium and strontium nitrates are used to color fireworks and signal flares; bismuth nitrate is used in making pharmaceuticals. Most nitrates are soluble in water, and a major use of nitric acid is to produce soluble metal nitrates. All nitrates decompose when heated and may do so explosively. The presence of nitrates in the soil is of great importance, since it is from these compounds that plants obtain the nitrogen necessary for their growth. Organic nitrates are esters formed by reaction of nitric acid with the hydroxyl (-OH) group in an alcohol. Nitrocellulose (or cellulose nitrate) is a highly flammable compound formed when cellulose materials are treated with concentrated nitric acid. The extent of the reaction between the cotton and acid can be varied to give a range of compounds, from the highly explosive gun-cotton to the flammable collodion cotton or pyroxilin. These are now used worldwide as propellants in cartridges and other ammunition. Collodion cotton and other less reactive forms of nitrocellulose are used chiefly in lacquers. They also form the basis of one of the earliest plastics, celluloid, made by the action on nitrocellulose of a solution of camphor in ethanol. Guncotton, fully nitrated cellulose, is used for explosives. Nitroglycerin is the nitric acid triester of glycerol and is more correctly called glycerol trinitrate. It is mixed with an absorbent material to form dynamite and is also used as a component of smokeless powder. Guncotton, fully nitrated cellulose is used for explosives. |
||