| AMMONIUM CHLORIDE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 12125-02-9 |
|
| EINECS NO. | 235-186-4 | |
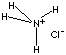
| FORMULA | NH4Cl | |
| MOL WT. | 53.49 | |
|
HS CODE |
2827.10.0000 | |
| TOXICITY | Oral rat LD50 : 1650 mg/kg | |
| SYNONYMS | Ammoniac; Ammonium Muriate; Sal ammoniac; | |
| Amchlor; Darammon; Salammonite; Salammoniac; Ammoniumchloridefume; Ammoniumchlorid (German); Chlorammonic (French; Chlorid Ammonia (Czech);Chlorid Amonny; Chlorid Amonny (Czech); Cloruro De Amonio; Gen-diur (Spainish); Muriate of Ammonia; Ammonium chloride; Other RN: 15630-61-2, 20548-08-7,,50295-88-0, 55871-05-1, 75944-36-4, 89485-85-8, 89485-84-7, 127634-24-6, 128532-42-3, 154383-48-9, 867060-75-1 | ||
| SMILES | [NH4+].[ClH-] | |
|
CLASSIFICATION |
|
|
|
EXTRA NOTES |
An acidifying agent that has expectorant and diuretic effects. Also used in
etching and batteries and as a flux in electroplating. The main application of ammonium chloride is as a nitrogen source in fertilizers |
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | Odourless, Colorless To White, Hygroscopic Solid | |
| MELTING POINT |
328 C |
|
| BOILING POINT | 520 C (sublimes) | |
| SPECIFIC GRAVITY | 1.53 | |
| SOLUBILITY | 28.3 g/100 ml at 25 C | |
| pH | 5.0 (10% sol at 25C) | |
| VAPOR DENSITY | 1.9 | |
|
AUTOIGNITION |
|
|
|
NFPA RATINGS |
Health: 21; Flammability: 0; Reactivity: 0 | |
|
REFRACTIVE INDEX |
||
| FLASH POINT | Not considered to be a fire hazard | |
| STABILITY | Stable under ordinary conditions | |
|
EXTERNAL LINKS & GENERAL DESCRIPTION |
||
|
Drug Information Portal (U.S. National Library of Medicine) - Ammonium chloride PubChem Compound Summary - Ammonium chloride KEGG (Kyoto Encyclopedia of Genes and Genomes) - Ammonium chloride http://www.ebi.ac.uk/ - Ammonium chloride http://www.ncbi.nlm.nih.gov/ - Ammonium chloride Human Metabolome Database - Ammonium chloride http://www.chem.unep.ch/ Local: |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
White Solid | |
|
ASSAY |
98.0%
min
|
|
|
MOISTURE |
1.0% max |
|
|
IRON |
50ppm max |
|
|
SULPHATES |
0.1% max |
|
|
pH |
4.5 - 5.2 |
|
|
WATER INSOLUBLES |
0.1% max |
|
| TRANSPORTATION | ||
| PACKING |
25kgs, 50kgs in bag |
|
| HAZARD CLASS | 9 (Packing Group:III) | |
| UN NO. | 3077 | |
| SAFETY INFORMATION | ||
|
HAZARD OVERVIEW |
OSHA Hazards:Harmful by ingestion., Irritant |
|
|
GHS |
|
|
| SIGNAL WORD |
Warning |
|
|
PICTOGRAMS |
|
|
|
HAZARD STATEMENTS |
H302-H319 |
|
|
P STATEMENTS |
P305+P351+P338 |
|
| EC DIRECTIVES |
|
|
| HAZARD CODES |
|
|
|
RISK PHRASES |
22-36 |
|
|
SAFETY PHRASES |
22 |
|
PRICE INFORMATION |
||
|
|
||