PRODUCT IDENTIFICATION
H.S. CODE
CLASSIFICATION
Monomer, Diene, Hemiterpene
EXTRA NOTES
By-product
of C5 fraction.
Used in manufacture of ''synthetic'' rubber, butyl rubber; copolymer in
production of elastomers
Overall Carcinogenic Evaluation: Group 2B
UN1218 [Flammable liquid]
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
220 C
NFPA RATINGS
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking - Isoprene
Google Scholar Search - Isoprene
Drug Information Portal (U.S. National Library of Medicine) - Isoprene
PubChem Compound Summary - Isoprene
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Isoprene
http://www.ebi.ac.uk/chebi/ - Isoprene
http://www.ncbi.nlm.nih.gov/ - Isoprene
Human Metabolome Database - Isoprene
http://ntp.niehs.nih.gov/
Use:The
majority of isoprene produced commercially is used to make synthetic
rubber (cis-polyisoprene), most of which is used to produce vehicle
tires. The second- and third-largest uses are in the production
of styrene-isoprene-styrene block polymers and butyl rubber (isobutene-isoprene
copolymer) (IARC 1994).
Production:Isoprene is recovered as a
by-product of thermal cracking of naphtha or gas oil from C5 streams
(IARC 1994, NTP 1999a). The isoprene yield is about 2% to 5% of
the ethylene yield. U.S. demand for isoprene grew 6.5% annually
from 1985 to 1992 (NTP 1999a). In 1994, isoprene production in the
United States was about 619 million pounds, almost 29% more than
in 1992. Estimated isoprene production capacity for eight facilities
was 598 million pounds in 1996, based on estimates of isoprene content
of product stream available from ethylene production via heavy liquids.
In 2009, isoprene was produced by 22 manufacturers worldwide, including
12 U.S. producers (SRI 2009), and was available from 23 suppliers,
including 12 U.S. suppliers (ChemSources 2009). U.S. imports of
isoprene (purity �� 95% by weight) increased from zero in 1989 to
a peak of 144 million pounds in 2003. Imports declined to 19.6 million
pounds in 2004, the lowest level since 1992, but remained near 32
million pounds from 2005 through 2008. During this period, U.S.
exports of isoprene ranged from 7.9 million to 39.6 million pounds
(in 2006) (USITC 2009). Reports filed from 1986 to 2002 under the
U.S. Environmental Protection Agency��s Toxic Substances Control
Act Inventory Update Rule indicated that U.S. production plus imports
of isoprene totaled 100 million to 500 million pounds (EPA 2004).
http://pages.towson.edu/
ISOPRENE
AND TERPENES: Some of the most interesting naturally occurring compounds
originate from 2-methyl-1,3-butadiene, otherwise known as isoprene,
in plants; the isoprene itself is synthesized from acetate. Pyrophosphate
esters of isoprene are the actual reagents for these construction
projects; pyrophosphoric acid is an anhydride of phosphoric acid.
isoprene isopentenyl pyrophosphate geranyl pyrophosphate --->
---> squalene The isoprene units are always linked 1,4 and head-to-tail
in terpenes (the preferred addition orientation even in mineral
acid), but are often linked further in bizarre ways to produce rings.
Oxygen functional groups are often included, as might be expected
from hydrolysis of the pyrophosphate linkage. The diversity of compounds
produced is amazing, but the pattern of one methyl group every fourth
carbon reveals their origin. The simplest, monoterpenes, consist
of 2 isoprene units. The stereoisomers of these simplest terpenes
provide interesting illustrations of the stereospecificity of odor
receptors; for example (+)-(S)-carvone is responsible for the odor
of caraway and (-)-(R)-carvone the odor of spearmint.
Local:
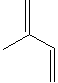
Isoprene, derived from butadiene, is a monomer which
contains two double bonds, and is
readily polymerized. It is a clear liquid; insoluble in water; soluble in
many organic solvents; boiling at 34 C. It is used in the manufacture of
polyisoprene synthetic rubbers and elastomers. Isoprene unit (CH2=C(CH3)-CH=CH2)
is abundant in nature. Natural rubber is a polymer of isoprene. Synthetic
polyisoprene rubber is the similarity of natural resin. The term terpene refers to a class of
naturally occurring compounds whose carbon skeletons are composed exclusively of
isoprene. Terpenes are major components of natural resin and some biological
compounds such as vitamins, carotenes and squalene. Isoprene monomer production
is almost entirely from C5 fraction as a by-product
and consumption is linked almost entirely to polyisoprene synthetic rubbers.
APPEARANCE
CONTENT
99.3% min
WATER
0.2% max
DIMER
0.3% max
PENTADIENE
100ppm max
ALKYNES
50ppm max
PARAFFINS
50ppm max
CYCLOPENTADIENE
10ppm max
SULFUR
5ppm max
TBC
100ppm (4-TERT-BUTYLCATECHOL)
TRANSPORTATION
1218
HAZARD OVERVIEW
May cause respiratory tract irritation. Causes eye and skin irritation. Extremely flammable liquid and vapor. Vapor may cause flash fire. Target Organs: Eyes, skin
GHS
Danger
PICTOGRAMS



HAZARD STATEMENTS
H224 Extremely flammable liquid and vapor
H312 Harmful
in contact with skin
H341 Suspected of causing genetic
defects
H350 May cause cancer
H412 Harmful to
aquatic life with long lasting effects
PRECAUTIONARY STATEMENTS
P201 Obtain special instructions before use
P210 Keep
away from heat/sparks/open flames/hot surfaces. - No smoking
P240
Ground/Bond container and receiving equipment
P243 Take
precautionary measures against static discharge
P273 Avoid
release to the environment
P280 Wear protective gloves/protective
clothing/eye protection/face protection
P281 Use personal
protective equipment as required
P302 + P352 IF ON SKIN:
Wash with plenty of soap and water
P308 + P313 IF exposed
or concerned: Get medical advice/attention
P403 + P235 Store
in a well-ventilated place. Keep cool
![]() F+
Extremely flammable
F+
Extremely flammable![]() T
Toxic
T
Toxic
RISK PHRASES
12 Extremely flammable
45 May cause cancer
52/53
Harmful to aquatic organisms, may cause long-term adverse
effects in the aquatic environment.
68 Possible risks of
irreversible effects.
SAFETY PHRASES
53 Avoid exposure - obtain special instructions before
use
45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where possible)
61
Avoid release to the environment. Refer to special instructions
/ safety data sheets.