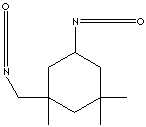
| ISOPHORONE DIISOCYANATE | |||||||||||||||||||||||||||||||||||||
|
PRODUCT IDENTIFICATION |
|||||||||||||||||||||||||||||||||||||
| CAS NO. | 4098-71-9 |
|
|||||||||||||||||||||||||||||||||||
| EINECS NO. | 223-861-6 | ||||||||||||||||||||||||||||||||||||
| FORMULA | (CH3)2C6H7(CH3)CH2(NCO)2 | ||||||||||||||||||||||||||||||||||||
| MOL WT. | 222.29 | ||||||||||||||||||||||||||||||||||||
| H.S. CODE | |||||||||||||||||||||||||||||||||||||
|
TOXICITY |
|||||||||||||||||||||||||||||||||||||
| SYNONYMS | IPDI, Methylene (3,5,5-trimethyl-3,1-cyclohexylene) ester; | ||||||||||||||||||||||||||||||||||||
| Isocyanic acid, methylene(3,5,5-trimethyl-3,1-cyclohexylene) ester; IPDI; Isocianato de 3-isocianatometil- 3,5,5-trimetil ciclohexilo; 3-Isocyanato methyl-3,5,5-Trimethyl cyclohexylisocyanate; 5-isocyanato- 1-(isocyanatomethyl)-1,3,3-trimethyl cyclohexane; 3-Isocyanatomethyl-3,5,5-trimethyl cyclohexylisocyanat; Isocyanate de 3-isocyanato méthyl-3,5,5-triméthylcyclohexyle; | |||||||||||||||||||||||||||||||||||||
|
DERIVATION |
|
||||||||||||||||||||||||||||||||||||
|
CLASSIFICATION |
|
||||||||||||||||||||||||||||||||||||
|
PHYSICAL AND CHEMICAL PROPERTIES |
|||||||||||||||||||||||||||||||||||||
| PHYSICAL STATE |
clear to pale yellow liquid, camphor like odor |
||||||||||||||||||||||||||||||||||||
| MELTING POINT | - 60 C | ||||||||||||||||||||||||||||||||||||
| BOILING POINT | 216 C | ||||||||||||||||||||||||||||||||||||
| SPECIFIC GRAVITY | 1.06 | ||||||||||||||||||||||||||||||||||||
| SOLUBILITY IN WATER | |||||||||||||||||||||||||||||||||||||
| pH |
|
||||||||||||||||||||||||||||||||||||
| VAPOR DENSITY | |||||||||||||||||||||||||||||||||||||
| AUTOIGNITION |
430 C |
||||||||||||||||||||||||||||||||||||
| NFPA RATINGS | Health: 3; Flammability: 1; Reactivity: 2 | ||||||||||||||||||||||||||||||||||||
|
REFRACTIVE INDEX |
|
||||||||||||||||||||||||||||||||||||
| FLASH POINT |
155 C |
||||||||||||||||||||||||||||||||||||
| STABILITY | Stable under ordinary conditions, | ||||||||||||||||||||||||||||||||||||
|
APPLICATIONS |
|||||||||||||||||||||||||||||||||||||
Diisocyanates (or polyisocyanates) are monomers for polyurethane production.
Polyurethane is made from a variety of diisocyanates in conjunction with
polyether and polyester polyols as co-reactants by addition polymerization which
needs at least two -N=C=O groups. Polyurethanes are widely used in the
manufacture of flexible and rigid foams, fibres, coatings, and elastomers. The
most common diisocyantes for this reaction are:
|
|||||||||||||||||||||||||||||||||||||
| SALES SPECIFICATION | |||||||||||||||||||||||||||||||||||||
|
APPEARANCE |
clear to pale yellow liquid |
||||||||||||||||||||||||||||||||||||
|
PURITY |
99.0% min |
||||||||||||||||||||||||||||||||||||
|
CHLORIDES |
0.05% max |
||||||||||||||||||||||||||||||||||||
|
COLOR, APHA |
30 max |
||||||||||||||||||||||||||||||||||||
| TRANSPORTATION | |||||||||||||||||||||||||||||||||||||
| PACKING | 200kgs in drum | ||||||||||||||||||||||||||||||||||||
| HAZARD CLASS | 6.1 (Packing group: III) | ||||||||||||||||||||||||||||||||||||
| UN NO. | 2290 | ||||||||||||||||||||||||||||||||||||
| OTHER INFORMATION | |||||||||||||||||||||||||||||||||||||
| Hazard Symbols: T, Risk Phrases: 23-36/37/38-42/43, Safety Phrases: 26-38-45 | |||||||||||||||||||||||||||||||||||||
|
GENERAL DESCRIPTION OF CYANATE (ISOCYANATE) |
|||||||||||||||||||||||||||||||||||||
| Cyanic acid (also called fulminic acid) is an unstable (explosive), poisonous, volatile, clear liquid with the structure of H-O-C��N (the oxoacid formed from the pseudohalogen cyanide), which readily polymerizes to cyamelide and fulminic acid. Cyanuric acid (also called pyrolithic acid), white monoclinic crystal with the structure of [HOC(NCOH)2N], is the compound of polymerized cyanic acid. Cyanic acid hydrolyses to ammonia and carbon dioxide in water. Its salts and esters are cyanates (or called fulminates). Esters of normal cyanic acid are not known. There is another isomeric cyanic acid with the structure of H-N=C=O, which is called isocyanic acid. Its salts and esters are isocyanates. Cyanates (or Isocyanates) are used in the manufacturing pharmaceuticals, pesticides, textile softener, lubricants and industrial disinfectants through the conversion to polycyclic compounds (such as hydantoins and imidazolons) They are used as plastic additives and as heat treatment salt formulations for metals. | |||||||||||||||||||||||||||||||||||||